Section 21 – Meat, reproduction and maternal trait in sheep and goats
Background
This Section covers the performance recording of traits to be considered for the genetic and genomic improvement of growth, meat, reproduction and maternal abilities in sheep and goat. It covers some (not all) of the main individual animal characteristics important for having more productive and efficient breeding females as well offspring (lambs and kids). The Guidelines below assume that a planned breed improvement programme at a population level will be put in place to compare animals either.
- within a breed,
- between breeds, and/or at
- national or international levels.
The information provided is for use as a guideline only.
The guidelines are relevant to dual-purpose meat and wool, meat and milk, and milk and wool animal systems.
This report covers the definition of each trait, the reason for recording it and recommendations for performance recording. It also provides guidelines for the genetic model to be used for genetic evaluations, including typical genetic parameters.
In the following sub-sections, we consider:
- Growth traits
- Carcass and meat quality traits
- Reproduction and maternal traits
In each sub-section, we will give some general considerations, define the traits that may be collected or calculated, provide some understanding for the rationale for including the trait, genomic information and genetic parameter estimates for use in genetic evaluations.
The guidelines do not cover the organisation of a breeding program and the different locations where selection is made (on-farm, in a central test station) and the type of selection (mass selection, progeny test). They are focused on the traits collected in performance recording.
Growth traits
Introduction
Lamb/kids growth to weaning and post-weaning growth to slaughter is a key aspect of meat production systems. Adult size of sire and dam influence lamb and kid growth that follows the typical gompertz curve trajectory[1] to maturity. The optimum point on the growth curve for harvesting meat varies according to different international preference and is also determined in part, by the projected mature size of offspring to be slaughtered. For example, in the absence of any genetic selection, lambs/kids born to heavier mature weight sire and dams will reach the same level of maturity at an older age compared to lambs/kids born to low mature weight sires and dams.
General considerations and rationale
General considerations
Growth traits are normally recorded on-farm. A range of weights can be recorded, including live weight at birth, 30, 42 to 120 days, and post weaning lamb/kid growth (6-10 months), 12-month weight and adult pre-mating weights. Adult weights can be recorded annually.
A complete set of weaning weights for all live animals should be recorded as this information is useful to infer survival, ewe/doe maternal effects and to account for selective culling of animals prior to recording later trait measurements.
For lambs and kids, the following data should be recorded:
- Unique identification of lamb/kid (usually includes Flock/Herd, Tag and Birth Year information, may include Country and Breed).
- Identification of the genetic sire and dam and rearing dam (if different to the genetic dam).
- Whether or not the lamb/kid was born as a result of Artificial Insemination (AI) or Embryo Transfer (ET)1.
- Recorded birth date or estimated birth date (based on foetal age at pregnancy scanning (ideally in 7 day intervals) or age (days) at weighing event).
- Birth rank and rearing rank (litter size), age of dam and any other associated data such as fostered1, hand reared.
- If lambs/kids are born dead or die within 24hrs of birth, these lambs/kids should be given a nominal identity for the purpose of recording this in the database (so that mortality can be captured and linked back to the genetic sire and dam).
- Gender of the lamb/kid, date of castration if applicable.
- Birth flock/herd, rearing flock/herd, current flock/herd.
- Recording date of live weight record.
- Management mob groups (e.g. grazing group) [relates to the animal management in the period prior to measurement].
- Death (or culling) date and reason.
- Carcass weight.
- Date of each measurement and scorer responsible.
Where animals have been managed differently in the period leading up to measurement, a management group should be assigned. It is critical when calculating between-group environmental effects to have effective genetic links between groups. An effective genetic link is usually considered as 20 measured progeny of a common sire in both management groups. Depending on the size of the flock/herd structure in the breeding programme, this figure may be revised to best match the realities on the ground.
There are several methods to examine the degree of connectedness and estimate the risk of comparisons of EBVs in across-flock/herd evaluation systems where natural mating takes place, such as follows: Connectedness Index[2], generalized Coefficient of Determination[3], average Prediction Error Variance of differences in EBVs between animals[4], variance of estimated differences between management unit effects[4], the Connectedness Rating[5], and Criterion of Admission to the group of Connected herds[6]. These methods mainly rely on having offspring from a common sire represented in the different management groups.
1This is to ensure that a distinction can be made between the genetic sire and dam, the dam carrying the lamb/kid and the rearing dam (if different from the carrying dam).
Rationale
Live weights of lambs and kids at different ages and rate of growth between them form the basis for comparing productivity, output and farm efficiency according to different management and grazing systems. Birth weights can impact positively or negatively on survival at birth. Growth to six weeks or weaning can inform maternal milking ability and direct lamb/kid growth to weaning and post-weaning impacts on time to processing, costs and feed consumed. Adult size is important in terms of efficiency, handling and stocking rate in maternal breeds.
Trait definitions
The traits are based on recording live weight at a given time point, usually at a given age or life event e.g. weaning. The definition of each weight differs with each country’s evaluation system. The trait can either be ‘live weight at a given age’ or ‘lamb/kid growth’ between two different ages. Additionally, an individual lamb/kid growth curve trajectory can be computed using all available live weight records over a defined period. This is more computationally intensive and to date, is not routinely used for large-scale genetic evaluations. Below are suggestions of weight at different age. The choice of age will depend upon each production system, and upon how much intensity of recording required.
- Live weight at birth - Measured in kg ideally at birth and prior to suckling. Should be measured on born alive and dead lambs/kids.
- Live weight at 30 days - Measured between 21 and 46 days (and subsequently analysed for 30 days).
- Live weight at 6 weeks - Measured between 20 and 65 days (and subsequently analysed for 42 days)
- Live weight at 100 days - Measured between 66 and 120 days (and subsequently analysed for 100 days)
- Live weight at 150 days - Measured between 121 and 180 days (and subsequently analysed for 150 days)
- Live weight at 30 days, 8 weeks, 100 days (or other ages) are possible
- Growth from birth to a defined age - Birth weight (within 24 hours of birth), 30 day weight for maternal recording, weight at weaning (42 to 120 days of age depending on system).
- Post weaning lamb/kid growth rate - Weights from weaning to 12 months of age.
- Adult live weight - Adult weights recorded annually at pre-mating. Can be considered as repeated measures trait.
- Number of days to slaughter- Time in days, from date of birth to slaughter date.
- Pre-slaughter live weight – The last live weight recorded on the animal before it is sold for slaughter.
The above ages of live weight are usual and must be considered as informative. Nevertheless, each country x breed x system may have its own relevant age to undertake weighing to fit into the system.
Animals should be treated consistently at weighing events (consistency across a management mob should be considered as well). They must be weighed at approximately the same time of day. Except for birth weight, live weight measurements should only be evaluated after a fasting period of a minimum of 2 hours (off feed and water).
Live weight should be evaluated to at least the nearest 0.5 kg (the nearest 0.1 kg for birth weight).
Note: all weigh scales (including fleece and live weight scales) should be calibrated at the start of each weighing session to ensure they are accurate. To calibrate scales, weigh a known standard weight three times to ensure the recorded weight is consistently accurate. The standard weight should be of similar weight to the fleeces of animal to be weighed. Before calibrating the scales, ensure the tare adjustment is set to zero. If the scale reading differs from the standard weight by more than 0.1 kg (fleece weight or birth weight) or 0.5 kg (later live weight), adjust or repair the scale until the required level of accuracy is achieved.
An example of computation of weight at standardised age and of growth is given in annex A.
It is generally thought that growth rate is polygenic (under the control of many genes with small additive effect). To date, no breeding programme includes any major gene effects for growth rate.
Genetic evaluation
Model of evaluation
The genetic model used to evaluate growth traits should use an ‘Animal’ model i.e. using pedigree information that includes information for sire and dam for each animal. Maternal genetic effect should be accounted for in the model if it is significantly different from zero. Scaling/adjustments are required for different means across contemporary groups prior to analysis.
Usual and recommended fixed effects are listed in the table 1 below, which below gives a summary of guidelines for evaluation of live weight and growth. Sometimes breed is considered, depending on the evaluation.
| Table 1. Summary of evaluation for live weight and growth. | |||||
|---|---|---|---|---|---|
| Trait Name | Definition | Recording | Fixed effects analysis model | Random effects | Additional notes |
| Live weight at a given age
e.g. -Birth -8 weeks -Weaning -Post-weaning -12 months -Adult |
Measurement (e.g. kg) from a weighing device. | Weights may vary with each country’s evaluation system. | -Flock/herd
-Year of birth -Lambing/kidding period of dam -Sex -Age of dam -Birth and rearing rank -Parity of dam -Other management effect (-breed) |
-Animal
-Maternal genetic if significant - Maternal permanent environmental effect -permanent environmental for repeated records e.g. adult live weight -Sire x flock/herd x year -Genetic groups |
Adjustment made for different means across contemporary groups prior to analysis |
| Growth rate / live weight gain | Change in live weight between 2 time points (calculated from the weights at different time points). | Birth-mid lactation
Birth-weaning Post-weaning |
Not frequently used in evaluations | ||
| Table 2. Genetic parameters for growth traits. | ||
|---|---|---|
| Traits | Heritability | Coefficient of variation (%) |
| Birth weight | 0.15-0.21 | 14.2-19.2 |
| Weaning weight | 0.18-0.23 | 15.8-18 |
| Post weaning lamb/kid growth | 0.21-0.33 | 10.6-12.8 |
| Adult weight | 0.30-0.41 | 6-12.4 |
Genetic parameters
It is highly recommended to use the genetic parameters estimated in the population evaluated. Each country x population should use its own genetic parameters (genetic variances, permanent environment variances residual variances, covariances if multi-trait evaluations). If there is no reservoir of data available in order to establish these genetic variances, we would suggest using variances that have been published from other breeding programmes with similar management and flock/herd size structure until such time there is enough data to establish variances from within the breeding programme.
For information, a common range of genetic parameters is given below[7].
Recommended prerequisites for recording of the animal
See list in section 1.1 “General considerations and rationale”.Meat traits
General considerations and rationale
Production of meat is the main product from sheep in many countries and a significant source of income from sheep and goat farming. As carcass traits are expressed post-mortem, predictors are used in vivo for carcass quality that are correlated to economically important post-slaughter measurements. These normally reflect what farmers are actually paid for.
Scanning technologies
There are several tools used for the prediction of carcass and meat quality. Currently, scanning technologies can be used to predict meat yields and fatness in live animals. These include ultrasound (US) and computer tomography (CT). Ultra-sound scanning is relatively inexpensive on a per-animal basis but is moderately accurate at predicting the weight of muscle and fat in the carcass. CT scanning is a very precise method (up to 96% accurate) and is an expensive tool. CT is usually used as a two-stage selection tool with animals pre-screened first, using ultrasound.
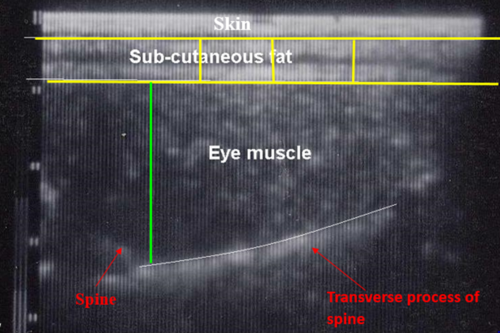
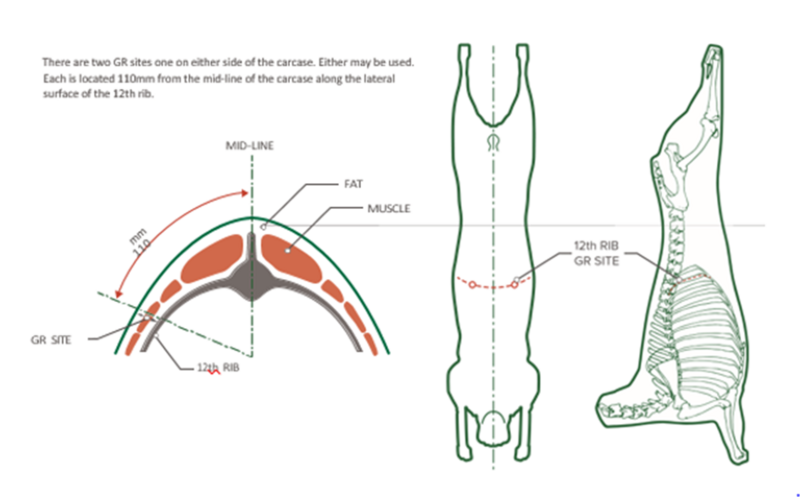
Measurements (mm) are taken of subcutaneous fat over the longissimus dorsi muscle at a prescribed site or sites typically the 3rd lumbar vertebrae (UK and Ireland) or between the 12th and 13th rib (Australia and New Zealand) by a trained accredited ultra-sound scanner. Scanner operators should be accredited and regularly calibrated, preferably against CT measurement or the carcass. An ultrasonic probe is used on a parted wool or shorn site primed by oil to generate an enhanced signal. A live weight measured at the time of scanning needs to be recorded to correct for live weight differences. Measurements should be recorded when there is variation between animals – typically greater than 35 kg live weight with a minimum of 2-3 mm or more of measured fat cover, depending on the breed.
Measurements recorded normally relate specifically to the identification of high-priced meat cuts that occur in the loin and gigot regions e.g. eye muscle (longissimus dorsi) dimensions -width and depth (mm), eye muscle area (cm2), fat depth (mm) measured at several different points above the eye muscle (and averaged) and 2-dimensional gigot muscularity. There is now scope for 3D muscularity to be used (routine use in the UK).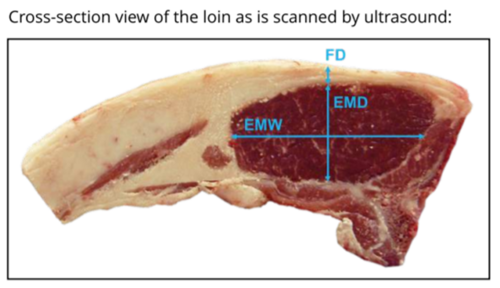


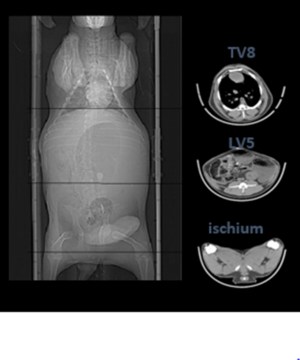
Computer tomography (CT) measurements (see figure 3) can be made on live or dead animals. Accuracy of measurement depends in part, on the frequency of scan slices across the carcass estimates of fat and muscle are typically over 95% accurate. New technology now enables ‘spiral scanning’ to take place, whereby slices of 5mm can be taken continuously throughout the body (like a spring), and then computer re-construction generates the relevant body part in real time, for analysis. Using strategic CT ‘slices’, fewer scans can be used to inform total carcass fat and lean and bone. A higher number of scans can be used to inform the distribution of fat and lean across the carcass regions informing breeding values for key carcass areas such as forequarter/shoulder, loin/saddle and hindquarter or gigot (hindleg) muscle dimensions. Also, additional measurements such as spine length, spine number and intra-muscular fat content (IMF) can be recorded. As CT is so highly accurate, it can be used to calibrate other meat measurements e.g. ultra-sound, carcass video and x-ray technologies or processor cutting systems.
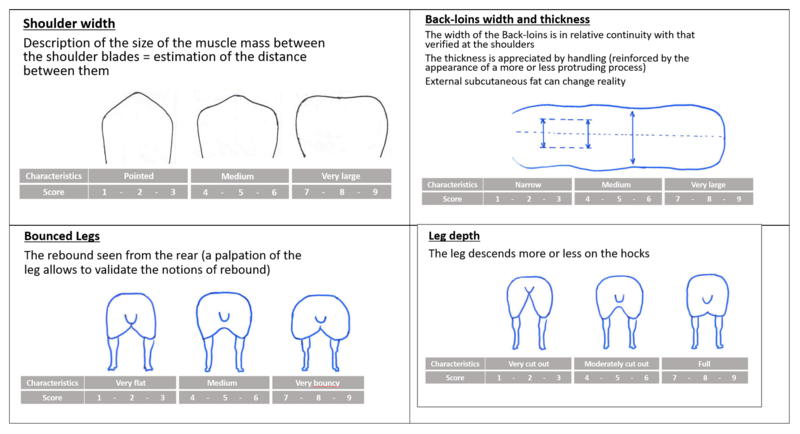
Linear scoring
In addition (or as an alternative method) to ultrasound and computer tomography, linear scoring may be used to assess the carcass and meat quality on live animals. The scoring is often made through a scale of notes ranging from 1 to 10 (for example). It may concern the following criteria: shoulders width, rump width, back-loins, legs (figure 4). Shoulders width, rump width and depth, back-loins, gigot muscularity, are proxies for assessing muscularity. A trained operator is needed to do the scoring correctly. Yearly sessions of training and harmonization between operators are required to maintain the quality of scoring.
Direct Carcass Measurements
Carcass weight and fat cover over the 12th rib (GR) can be recorded at processing. A number of other measurements such as video scan and x-ray technologies can be used if calibrated against carcass dissections or spiral computer tomography.
Measures of meat quality such as intra-muscular fat, marbling score, pH, and colour can also be recorded on carcasses using NIR technology.
Reporting hot vs cold weights at abattoir - Hot carcass weight is the weight of the carcass once it reaches the end of the processing line. The cold carcass weight is recorded 24 hours after chilling. These two weights have a highly significant correlation. The key thing is to ensure that there is consistency in exactly what is being recorded. .
Timing of live weight recording to get KO% - Ideally, animals should be weighed on the day of slaughter after a period of fasting to remove the variation in gut fill.
Novel traits (meat science, sensory)
Other traits, not routinely taken into account, might be measured / assessed: sensory traits (Juiciness, Flavour, Tenderness, Overall Liking), nutrient content (Iron, Zinc), Amino Acids (Omega 3), colour (Fresh / Aged / Retail), Ph.
These traits are mentioned for information, but as they are too expensive to measure, no selection index include them so far.
Rationale
For meat-focused sheep/goat production systems – meat is the final product for consumption. Financial returns to the producer are determined by carcass weight, confirmation and fatness. Meat and fat are both heritable and variable, allowing for genetic improvement in these traits.
Trait Definition
Merit for meat can be expressed in a number of ways depending on the evaluation:
- (Cold)Carcass weight: weight of the empty carcass informed by growth and meat yield information in live animals or measured carcass weight on processed animals.
- Fat Depth: describe the merit for fat depth at a constant weight. A negative value means a genetically less fat animal, a positive or higher value a fatter animal at a constant carcass weight.
- Fat Yield: proportion of the carcass kg of fat per kg of carcass weight at a constant weight.
- Lean Yield: kg of Lean (muscle) tissue per kg of carcass weight at a constant weight.
- Eye Muscle Depth: describes the merit for eye muscle depth at a constant weight. A positive value indicates a higher proportion of the carcass as lean muscle and indicates more lean tissue in the higher priced cuts.
- Eye Muscle Area: describes the merit for eye area at a constant weight. A positive value indicates a higher proportion of the carcass as lean muscle and indicates more lean tissue in the higher priced cuts.
- Yield by Carcass region: if data available to discriminate between animals for fat and lean tissue across main carcass regions; Shoulder, Loin, Hindquarter or Gigot (large leg muscle) at a constant carcass weight.
- Dressing out % (or killing out % or KO%): percentage of cold carcass weight related to the fasted liveweight of the animal prior to slaughter.
- Days to Slaughter: age at slaughter
Several major genes have been discovered in sheep breeds which impact on meat traits, such as:
- Callipyge - increased muscling of the pelvic limbs and loin.
- Carwell - increased loin muscle weight and dimension.
- GDF8 – associated with increased muscle and decreased fat due to myostatin effects.
- BCO2 – yellow fat gene inherited recessive trait[8].
Other major genes should be discovered or put in evidence soon. That is why this list is not comprehensive. For any reference, see the generic reporting in OMIA (Online Mendelian Inheritance in Animals) which is a catalogue/compendium of inherited disorders, other (single-locus) traits, and genes in animal species.
Genetic evaluation
Model of evaluation
The genetic model used to evaluate meat traits should use an ‘Animal’ model i.e. using pedigree information that includes information for sire and dam for each animal. Maternal genetic effect should be accounted for traits if they are significant. Scaling/adjustments are required for different means across contemporary groups in the analysis.
The table below gives a summary of guidelines for evaluation of ultrasonic fat and muscle, computer tomography traits and carcass traits. This summary includes usual and recommended fixed effects.
| Table 3. Summary of evaluation for ultrasound, computer tomography (CT) and carcass traits | |||||
|---|---|---|---|---|---|
| Trait Name | Definition | Recording | Fixed effects analysis model | Random effects | Additional notes |
| Ultrasonic fat and muscle depth on live animal | Measurement (mm) of subcutaneous fat over the longissimus dorsi at a prescribed site or sites e.g. 3rd lumbar vertebra, or between 12-13th rib.
Muscle is recorded as depth, width or area of the longissimus dorsi measured at the same site. |
Ultrasonic probe used on parted wool site primed by oil to generate enhanced signal by trained operator. | -Flock/Herd
-Birth year -Sex -Age of dam -Birth and rearing rank -Age at measurement -Management (grazing) mob (-Breed)
|
-Animal
-Sire x flock/herd year -Genetic groups |
Ultrasound operators should be accredited and calibrated regularly, preferably against gold standard of CT.
A live weight should be taken at the point of ultrasonic scanning. Timing of ultrasonic scanning should be when there is sufficient expression of variability in this trait. |
| CT | Gigot muscularity
IMF Spine length Spine number CT lean CT fat CT bone Fore quarter/ shoulder Loin/Saddle Hindquarter |
||||
| Carcass traits | Hot/Cold carcass weight
Fat & Muscle depths/ area/width Dressing /KO% Cut weights (of calibrated) |
-Animal
-Sire x flock/herd year -Genetic groups |
Pre-slaughter weight needed for KO% | ||
Genetic parameters
It is highly recommended to use the genetic parameters estimated in the population evaluated. Each country x population should use its own genetic parameters (genetic variances, permanent environment variances residual variances, covariances if multi trait evaluations).
For information, usual genetic parameters are given below[9] (French parameters).
| Table 4. Genetic parameters for meat traits. | ||
|---|---|---|
| Traits | Heritability | Coefficient of variation (%) |
| Carcass weight | 0.20-0.30 | 17.6 |
| Fat depth – live, adjusted weight | 0.25 | 12.7 |
| Fat depth - carcass C site | 0.30 | 46.2 |
| Fat depth - carcass GR site | 0.32 | 40.1 |
| Eye muscle depth – live, adjusted weight | 0.22 | 10.8 |
| Eye muscle width – live, adjusted weight | 0.04 | 6.3 |
| Eye muscle area – live, adjusted weight | 0.14 | 7.9 |
| Eye muscle depth – carcass | 0.30 | 11 |
| Eye muscle width – carcass | 0.38-0.50 | 6.9 |
| Eye muscle area – carcass | 0.41 | 13.8 |
| Lean meat yield – live, adjusted weight | 0.35 | 9 |
Recommended prerequisites for recording of the animal
- Breed.
- Birth/ rearing rank.
- Birth flock/herd / rearing flock/herd.
- Sire.
- Dam Genetic.
- Dam Rearing.
- AI / ET use.
- Sex.
- Date castrated (if applicable).
- Age of dam.
- Date of birth and weigh date or age at weighing occasion.
- Management mob groups (e.g. grazing groups).
- Death date and reason.
- Date of measurement and operator name/code.
Reproduction, maternal and lamb/kid survival traits
Maternal traits are typically either: 1. Measured directly on breeding females, 2. Measured on breeding males, or 3. Measured on offspring directly, and 4. Measured on offspring and the maternal genetic variance apportioned accordingly.
- Ultrasonic scanning no. lambs/kids (1).
- Number of lambs/kids born alive (1).
- Number of lambs/kids born dead (1).
- Number of lambs/kids reared (to a defined end point) (1).
- Lamb/kid survival to a defined age or lamb/kid live weight weighing occasion (1, 3, 4).
- Scrotal circumference (2).
- Maternal lambing/kidding difficulty (1).
- Ewe/doe milk yield (1) or estimated from the lamb/kid growth rate as a proxy trait for milk yield to a defined point in time) (3).
- Ewe/doe survival (1).
General considerations and rationale
General considerations
Reproduction and maternal traits are recorded on-farm. For some information (e.g. pregnancy scan information), data are collected by trained technicians. Survival of lambs/kids born alive can be inferred from data collected at birth and at later live weight recording occasions. Alternatively, lamb/kid survival as a trait of the dam can be attributed from the difference between the number of foetuses scanned at mid-pregnancy and the number of lambs/kids reared by the dam to a given end-point (later recording occasion).
All the lambing/kidding data from animals to be kept as breeding animals must be collected. That assumes that all such animals kept for breeding are entered onto the database will be used for genetic evaluations. For some countries there is also a requirement for them to also be registered with their breed society identification (ID) number. The inventory of the flock/herd must be held and kept up to date (IDs of new animals to be bred and IDs of animals being culled or sold from the recorded flock/herd).
For the dam affiliation, the following data should be recorded (data relative to lambing/kidding and birth collected by farmers):
- ID of the genetic dam
- ID of the rearing dam (if different to a). Note that if the rearing dam is not one of the performance-recorded flock/flock, and has no formal identity, then a dummy number should be assigned, as well as the breed and age of rearing dam recorded.
- Date of lambing/kidding.
- ID of the lambs/kids. Note that a dummy number should be assigned to born-dead lambs/kids so that birth weights and gender can be attributed to these lambs/kids and siblings.
- Gender of the lambs/kids born alive and dead.
For the sire affiliation, the following data should be recorded at mating:
- ID of the sire
- Date a male was introduced to the flock/herd, date it was taken out. Any subsequent males used recorded in the same manner. These should be keel-marked to identify which ewes/does will give birth to rams/bucks from different sires and to ensure the parentage of lambs/kids that could be from either sire.
- Method of reproduction (insemination or natural mating)
Rationale
Reproduction traits are recorded for the genetic improvement of meat sheep because:
- they are a driver of the revenue of the farm (e.g. prolificacy);
- there may be antagonistic correlated responses to fertility from only including growth and carcass traits in sheep breeding programmes, and
- several of the recorded fertility characteristics are used in the genetic model for breeding value estimation (e.g. the litter size a lamb/kid was born into.)
Prolificacy (number of lambs/kids born) mainly depends on the ovulation rate and embryonic survival.
The number of lambs/kids reared depends on both prolificacy and lamb/kid survival from birth to a given end-point. For example, the number of lambs/kids reared to 8 weeks of age.
Key flock/herd efficiency indicators often include the following two definitions:
- Number of lambs/kids born (dead + alive) per number of females put to the sire(s).
- Number of lambs/kids born (dead + alive) per female lambing/kidding.
Using ultrasonic pregnancy scanning is a good indicator for fertility as it counts the number of foetuses being carried per female at mid-pregnancy. This information can then be used to allocate appropriate nutrition in the latter stages of pregnancy, according to the number of ewe lambs/doe kids are carrying.
The age at first lambing/kidding may give information on the precocity of the dam although it is heavily influenced by flock/herd management decisions. For example, a breeder may decide not to mate any ewe lamb /doe kid that has reached less than 60% of mature live weight at the time of possible first mating opportunity.
For the same reason, the lambing/kidding interval (defined as being the difference between subsequent lambing/kidding occasions) may also be an indicator of female fertility although, as many sheep have strong seasonal anoestrus, the actual time-lag is mostly an indication of ‘missed mating years’ in the case of once/year lambing/kidding systems.
Artificial synchronization, use of hormonal treatments or exposure to ‘teaser’ males pre-mating and AI and ET regimes all affect fertility & quality of ovulation.
For this reason, to compare such animals like-for-like for fertility, it is important that knowledge of the farming system and breeding practices are captured within the planned breeding programme. By so doing, this information can then be ‘adjusted for’ using the knowledge of management type.
Trait definition
Ultrasonic number of lambs/kids carried at mid-pregnancy: it is normally undertaken at ~day 70 of pregnancy (60-90d). The presence /absence of pregnancy can be recorded, but most useful is the number of lambs/kids carried by the pregnant dam. This may be 0,1,2,3, 4 or more. Additional information on stage of gestation can be collected (e.g. to lamb/kid early/ late in relation to the specified start of lambing/kidding date).
Number of lambs/kids born alive or dead: it is the number of lambs/kids born (alive and dead) when pregnancy reaches full term. The other terms used are litter size at birth or prolificacy. The values may be 1,2,3,4, or more. Lambs/kids that died soon after (within 24hrs) birth must be recorded (identified with a number) as dead. Abortions can be recorded as ewes/does ‘not lambing/not kidding’. Sometimes it is difficult to know if ewes/does lambing/kidding prematurely, are abortions. Generally speaking, it is unusual for late abortions to occur and (in the case of extensively managed flocks/herds) sometimes aborted lambs/kids are not found.
Number of lambs/kids reared: it is the number of lambs/kids reared by the ewe/doe that are still alive at a given age. The age at which one considers the lamb/kid reared must be notified as an additional information. For example, the age can be determined by a weighing (for example at eight weeks of age). The values may be 1,2,3,4 or more.
Lamb/kid survival to a given age: it can be recorded as a dam trait (ie as the number of a dam’s own lambs/kids alive at a given age). The data can be generated by the presence or absence of a lamb/kid live weight recorded at the targeted age. It can also be recorded as a direct lamb/kid trait (i.e. attributing the lamb’s/kid’s own genes to whether or not they survive.). The trait can be analysed as a direct lamb/kid trait and the maternal genetic variance used as the maternal component of lamb/kid survival.
Lambing/kidding interval: it corresponds to the number of days between subsequent lambings/kidding.
Age at first lambing/kidding: it is the age of the dam at the first lambing/kidding.
Milking/maternal ability: this is also called ewe/doe mothering ability or milkability. A classical indicator is the live weight of offspring at around mid-lactation (30-50d) that represents the ability of the dam to produce enough milk for its offspring. An example of methods for estimating a standardized weight at 30 days is described below.
Lambing/kidding ease: score given by the farmer describing in which extent the birth of the lamb/kid was easy or not.
Vigour/activity of the lamb/kid at birth: score given by the farmer describing in which extent the young is active just after the birth.
Several major genes have been discovered in many sheep breeds since the initial discovery in Booroola Merinos (1982), which affect the ovulation rate, hence indirectly prolificacy. We can mention some of them:
• Merinos Booroola BMPR1B[10][11]
• BMP15 (X chromosome)
• Galway GDF9[12]
• FecL mutation (autosomal chromosome)[12]
• Thoka gene[13]
This list is not comprehensive and might evolve as other major genes are discovered or put in evidence soon. For any reference, see the generic reporting in OMIA (Online Mendelian Inheritance in Animals) which is a catalogue/compendium of inherited disorders, other (single-locus) traits, and genes in animal species.
Some sheep breeding programmes actively report the presence or absence of major genes for key aspects of production, health or efficiency. Some of these genes should be actively managed. For example, in some cases, if the hyper-prolific allele has a positive effect on heterozygous female, its effect is often too large (leading to high rates of neonatal lamb/kid mortality) or deleterious (due to fertility issues) in homozygous carrier females.
Genetic evaluation
Model of evaluation
Some countries consider lambing/kidding at 1 year old as a separate trait to that of subsequent ages.
Therefore, animals have two breeding values for fertility and also for number of lambs/kids born.
Animals can be grouped into contemporary groups which may include flock/herd, birth year, lambing/kidding season, grazing location.
Prolificacy
Litter size is a discrete trait; with good consistency between the underlying normal model and observed litter size frequency. Genetic evaluations can be evaluated on this underlying normal distribution scale. Threshold and distribution parameters are considered as known a priori (in practice, they are calculated for each breed according to the litter size frequency). Variable analysed is the normal score.
If a major gene is genotyped, it is recommended to take into account this information in the model, in order to estimate the polygenic variability better.
The genetic model includes the genetic value of the animal, the permanent environmental effect and fixed effects, among which:
- The breed of the sire and the dam
- The contemporary group defined as flock/herd*year*season and possibly grazing location
- The reproduction method and the insemination methods / embryo transfer
- Ewe’s/doe’s physiological condition at lambing/kidding (such as: parity/age lambing/kidding rank and number of lambs/kids weaned at previous lambing/kidding)
- The birth season for ewe/doe lambs/kids
Prolificacy on induced oestrus and prolificacy on natural oestrus are 2 different traits (genetic correlation around 0.75). When induction of oestrus is practiced in the population, it is possible to include both traits in the evaluation while considering a bivariate repeated measures animal model.
In this case, an index combining prolificacy on induced and natural oestrus may be published EBV, such as: EBVprolificacy = a EBVnatural oestrus + b EBVinduced oestrus
Maternal ability
For assessing maternal ability, two traits may be evaluated, in a bi-trait model:
- milking value (proxy = standardized weight at 30 days);
- young survival at weaning.
The phenotypes are measured on the lambs/kids.
The genetic model includes the genetic value of the animal, the permanent environmental effect and fixed effects, among which:
- The breed of the sire and the dam.
- The contemporary group defined as flock/herd * year * season.
- The suckling mode.
- The lambing/kidding mode (lamb/kid born simple, double, triple …).
- The breeding mode (lamb/kid bred simple, double, triple …).
- The number of male lambs/kids in the litter.
- The lambing/kidding interval.
- The age at first lambing/kidding.
- The lambing/kidding rank (parity).
- The number of lambs/kids weaned at previous lambing/kidding.
These effects can be combined or estimated as interaction.
For example, it is possible to consider the dam’s physiological status at lambing/kidding as a combination between lambing/kidding interval or age at first lambing/kidding, parity and number of lambs/kids weaned at previous lambing/kidding; it is possible to consider a combination between lambing/kidding mode and breeding mode.
An index combining milking ability and young survival at weaning may be published EBV, such as:
EBVmaternal ability = a EBVyoung survival at weaning + b EBVmilking ability
Genetic parameters
The main features of the polygenic variation are:
- Prolificacy has a low heritability (0.08 – 0.10) and a low repeatability (0.15 – 0.20) but a quite high coefficient of variation (35-45%)
- Genetic correlation between natural and induced oestrus: 0.75
The table below gives usual range for genetic parameters of reproduction and maternal traits.
| Table 5. Genetic parameters for reproduction traits. | |||
|---|---|---|---|
| Trait | Heritability | Repeatability | Coefficient of variation |
| Number of lambs/kids born | 0.08-0.11 | 0.15-0.20 | 35-40% in natural conditions; 40-45% after induced oestrus |
| Number of lambs/kids reared | 0.03-0.09 | ||
| Perinatal lamb/kid survival | 0.05 | ||
| Lamb/kid survival at weaning | 0.10 (direct effect)
|
0.30 | |
- ↑ Lambe, N.R., Navajas, E.A., Simm, G., Bünger, L. (2006). A genetic investigation of various growth models to describe growth of lambs of two contrasting breeds. J Anim Sci. 2006 Oct; 84(10):2642-54.
- ↑ Foulley J.L., Hanocq E., Boichard D. 1992. A criterion for measuring the degree of connectedness in linear models of genetic evaluation. Genet. Sel. Evol., 24: 315–330.
- ↑ Laloe D. 1993. Precision and information in linear models of genetic evaluation. Genet. Sel. Evol., 25: 557-576.
- ↑ 4.0 4.1 Kennedy B.W., Trus D. 1993. Considerations on genetic connectedness between management units under an animal-model. J. Anim. Sci., 71: 2341–2352.
- ↑ Mathur P.K., Sullivan B.P., Chesnais J.P. 2002. Measuring connectedness: concept and application to a large industry program. In Proc 7th World Congr. Genet. Applied to Livest. Prod., vol 32. Montpellier, pp 545–548.
- ↑ Mathur P.K., Sullivan B.P., Chesnais J.P. 2002. Measuring connectedness: concept and application to a large industry program. In Proc 7th World Congr. Genet. Applied to Livest. Prod., vol 32. Montpellier, pp 545–548.
- ↑ Safari, E., Fogarty, N.M. & Gilmour, A. R. (2005). A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Livestock Production Science, 92 (3), 271-289
- ↑ Våge, D.I. and Boman, I.A. 2010. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 2010 Feb 2;11:10.
- ↑ Safari, E., Fogarty, N.M. & Gilmour, A. R. (2005). A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Livestock Production Science, 92 (3), 271-289
- ↑ Piper, L. R. and Bindon, B. M. 1982. Genetic segregation for fecundity in Booroola Merino sheep. Proceedings of the world congress on sheep and beef cattle breeding, New Zealand (ed. Barton, R. A. and Smith, W. C.), vol. 1, pp. 395–400.
- ↑ 11.0 11.1 Laloe D. 1993. Precision and information in linear models of genetic evaluation. Genet. Sel. Evol., 25: 557-576.
- ↑ 12.0 12.1 Drouilhet, L., Lecerf, F., Bodin, L., Fabre, S., Mulsant, P. 2009. Fine mapping of the FecL locus influencing prolificacy in Lacaune sheep. Animal Genetics, Vol 40, issue 6, dec. 2009.
- ↑ 13.0 13.1 Russel, A., Alexieva, S., & Elston, D. (1997). The effect of the introduction of the Thoka gene for fecundity on lamb production from Cheviot ewes. Animal Science, 64(3), 503-507.
