Section 07 – Bovine Functional Traits: Difference between revisions
| (12 intermediate revisions by the same user not shown) | |||
| Line 121: | Line 121: | ||
| rowspan="2" style="text-align:left;" |'''Source of data''' | | rowspan="2" style="text-align:left;" |'''Source of data''' | ||
| colspan="2" style="text-align:center;" |'''Type of data''' | | colspan="2" style="text-align:center;" |'''Type of data''' | ||
|- | |- style="background-color:#efefef;" | ||
| style="text-align:center;" |'''Direct health information''' | | style="text-align:center;" |'''Direct health information''' | ||
| style="text-align:center;" |'''Indirect health information''' | | style="text-align:center;" |'''Indirect health information''' | ||
| Line 1,171: | Line 1,171: | ||
Government bodies and other organisations involved in animal health issues are very interested in monitoring the health status of the cattle population. Consumers also are increasingly concerned about aspects of food safety and animal welfare. Regardless of which sources of health information are used, national monitoring programs may be developed to meet the demands of authorities, consumers and producers. The latter may particularly benefit from increased consumer confidence in safe and responsible food production. | Government bodies and other organisations involved in animal health issues are very interested in monitoring the health status of the cattle population. Consumers also are increasingly concerned about aspects of food safety and animal welfare. Regardless of which sources of health information are used, national monitoring programs may be developed to meet the demands of authorities, consumers and producers. The latter may particularly benefit from increased consumer confidence in safe and responsible food production. | ||
Fertility data is also important for providing genetic evaluations, both within country and between countries. The following section is from the Interbull website ( | Fertility data is also important for providing genetic evaluations, both within country and between countries. The following section is from the Interbull website (http://www.interbull.org/ib/idea_trait_codes) and are the traits that the Interbull Steering committee chose in August 2007 to become part of MACE evaluations of fertility. Interbull considers female fertility traits classified as follows: | ||
# T1 (HC): Maiden (H)eifer's ability to (C)onceive. A measure of confirmed conception, such as conception rate (CR), will be considered for this trait group. In the absence of confirmed conception an alternative measure, such as interval first-last insemination (FL), interval first insemination-conception (FC), number of inseminations (NI), or non-return rate (NR, preferably NR56) can be submitted. | # T1 (HC): Maiden (H)eifer's ability to (C)onceive. A measure of confirmed conception, such as conception rate (CR), will be considered for this trait group. In the absence of confirmed conception an alternative measure, such as interval first-last insemination (FL), interval first insemination-conception (FC), number of inseminations (NI), or non-return rate (NR, preferably NR56) can be submitted. | ||
| Line 2,344: | Line 2,344: | ||
Nordic countries have pioneered the recording of claw health from claw trimming visits and then systematically using the data. Routine documentation of claw health data started in Sweden in 2003 and one year later in Finland and Norway (Johansson ''et al''. 2011<ref>Johansson, K., J.-Å. Eriksson, U.S. Nielsen, J. Pösö, and G.P. Aamand. 2011. Genetic evaluation of claw health in Denmark, Finland and Sweden. Interbull Bull. 44:224–228. </ref>, Ødegård ''et al''. 2013<ref>Ødegård, C., M. Svendsen, and B. Heringstad. 2013. Genetic analyses of claw health in Norwegian Red cows. J. Dairy Sci. 96:7274–7283. doi:10.3168/jds.2012-6509.</ref>, Häggman & Juga 2013<ref name=":0">Häggman, J., and J. Juga. 2013. Genetic parameters for hoof disorders and feet and leg conformation traits in Finnish Holstein cows. J. Dairy Sci. 96:3319–3325. doi:10.3168/jds.2012-6334.</ref>). Since 2006 claw health data has been routinely recorded in the Netherlands. In several countries it is now possible to electronically register data from claw trimming visits and recording systems and consequently accessibility of claw data have improved. Electronic systems by professional trimmers to document claw health status are,for example, used in Denmark, Finland, Sweden, Norway, Canada, France, Germany, and Spain (Kofler, 2013<ref>Kofler, J., 2013. Computerised claw trimming database programs as the basis for monitoring hoof health in dairy herds. Veterinary Journal 198, 358-361.</ref>). With this development, larger amounts of claw health data are becoming available, implying the need for harmonization and further measures to strengthen data quality and consistency. | Nordic countries have pioneered the recording of claw health from claw trimming visits and then systematically using the data. Routine documentation of claw health data started in Sweden in 2003 and one year later in Finland and Norway (Johansson ''et al''. 2011<ref>Johansson, K., J.-Å. Eriksson, U.S. Nielsen, J. Pösö, and G.P. Aamand. 2011. Genetic evaluation of claw health in Denmark, Finland and Sweden. Interbull Bull. 44:224–228. </ref>, Ødegård ''et al''. 2013<ref>Ødegård, C., M. Svendsen, and B. Heringstad. 2013. Genetic analyses of claw health in Norwegian Red cows. J. Dairy Sci. 96:7274–7283. doi:10.3168/jds.2012-6509.</ref>, Häggman & Juga 2013<ref name=":0">Häggman, J., and J. Juga. 2013. Genetic parameters for hoof disorders and feet and leg conformation traits in Finnish Holstein cows. J. Dairy Sci. 96:3319–3325. doi:10.3168/jds.2012-6334.</ref>). Since 2006 claw health data has been routinely recorded in the Netherlands. In several countries it is now possible to electronically register data from claw trimming visits and recording systems and consequently accessibility of claw data have improved. Electronic systems by professional trimmers to document claw health status are,for example, used in Denmark, Finland, Sweden, Norway, Canada, France, Germany, and Spain (Kofler, 2013<ref>Kofler, J., 2013. Computerised claw trimming database programs as the basis for monitoring hoof health in dairy herds. Veterinary Journal 198, 358-361.</ref>). With this development, larger amounts of claw health data are becoming available, implying the need for harmonization and further measures to strengthen data quality and consistency. | ||
The ICAR Claw Health Atlas<ref>ICAR Claw Health Atlas</ref> was published in 2015 (Egger-Danner ''et al''. 2015<ref>Egger-Danner, C., P. Nielsen, A. Fiedler, A. Müller, T. Fjeldaas, D. Döpfer, V. Daniel, C. Bergsten, G. Cramer, A.-M. Christen, K.F. Stock, G. Thomas, M. Holzhauer, A. Steiner, J. Clarke, N. Capion, N. Charfeddine, J.E. Pryce, E. Oakes, J. Burgstaller, B. Heringstad, C. Ødegård, J. Kofler, F. Egger, and J.B. Cole. 2015. ICAR Claw Health Atlas. ICAR Technical Series. No. 18. International Committee for Animal Recording, Rome, Italy.</ref>) and has so far been translated to nineteen languages. The aim of this atlas was to harmonise the collection of high quality data within and across countries. | The [https://www.icar.org/index.php/publications-technical-materials/technical-series-and-proceedings/atlas-claw-health-and-translations/ ICAR Claw Health Atlas]<ref>ICAR Claw Health Atlas</ref> was published in 2015 (Egger-Danner ''et al''. 2015<ref>Egger-Danner, C., P. Nielsen, A. Fiedler, A. Müller, T. Fjeldaas, D. Döpfer, V. Daniel, C. Bergsten, G. Cramer, A.-M. Christen, K.F. Stock, G. Thomas, M. Holzhauer, A. Steiner, J. Clarke, N. Capion, N. Charfeddine, J.E. Pryce, E. Oakes, J. Burgstaller, B. Heringstad, C. Ødegård, J. Kofler, F. Egger, and J.B. Cole. 2015. ICAR Claw Health Atlas. ICAR Technical Series. No. 18. International Committee for Animal Recording, Rome, Italy.</ref>) and has so far been translated to nineteen languages. The aim of this atlas was to harmonise the collection of high quality data within and across countries. | ||
The purpose of these ICAR guidelines is to give recommendations on recording, data validation and use of claw health information, with focus mainly on claw trimming data. | The purpose of these ICAR guidelines is to give recommendations on recording, data validation and use of claw health information, with focus mainly on claw trimming data. | ||
| Line 2,362: | Line 2,362: | ||
|style="text-align:left;"|1 | |style="text-align:left;"|1 | ||
|style="text-align:center;"|Claw Trimming Data | |style="text-align:center;"|Claw Trimming Data | ||
|style="text-align:center;"|Several studies have shown that data recorded by hoof trimmers are suitable for genetic evaluation of claw health (Häggman & Juga 2013<ref name=":0" />; Koenig et al. 2005<ref>Koenig, S., A.R. Sharifi, H. Wentrot, D. Landmann, M. Eise, and H. Simianer. 2005. Genetic parameters of claw and foot disorders estimated with logistic models. J. Dairy Sci. 88:3316–3325. doi:10.3168/jds.S0022-0302 (05)73015-0.</ref>; van Pelt 2015<ref>Van Pelt, M.L. 2015. Implementation of a claw health index in The Netherlands. Page Seminar des Ausschusses für Genetik der ZAR, Salzburg, Austria.</ref>). Claw disorders are included in the comprehensive ICAR Central Health Key, that is consistent with the ICAR Standard for claw data recording and the ICAR Claw Health Atlas (see appendix of the ICAR Health guidelines). These standards should be referred to in electronic systems supposed to facilitate data recording in connection with claw trimming. | |style="text-align:center;"|Several studies have shown that data recorded by hoof trimmers are suitable for genetic evaluation of claw health (Häggman & Juga 2013<ref name=":0" />; Koenig et al. 2005<ref>Koenig, S., A.R. Sharifi, H. Wentrot, D. Landmann, M. Eise, and H. Simianer. 2005. Genetic parameters of claw and foot disorders estimated with logistic models. J. Dairy Sci. 88:3316–3325. doi:10.3168/jds.S0022-0302 (05)73015-0.</ref>; van Pelt 2015<ref>Van Pelt, M.L. 2015. Implementation of a claw health index in The Netherlands. Page Seminar des Ausschusses für Genetik der ZAR, Salzburg, Austria.</ref>). Claw disorders are included in the comprehensive ICAR Central Health Key, that is consistent with the ICAR Standard for claw data recording and the [https://www.icar.org/index.php/publications-technical-materials/technical-series-and-proceedings/atlas-claw-health-and-translations/ ICAR Claw Health Atlas] (see appendix of the ICAR Health guidelines). These standards should be referred to in electronic systems supposed to facilitate data recording in connection with claw trimming. | ||
The high coverage and regular structure of the claw trimming data make them highly valuable for analyses, and these guidelines will focus on that source of information on claw health. | The high coverage and regular structure of the claw trimming data make them highly valuable for analyses, and these guidelines will focus on that source of information on claw health. | ||
| Line 2,745: | Line 2,745: | ||
For the calculation of incidence and prevalence rates three important concepts should be defined: | For the calculation of incidence and prevalence rates three important concepts should be defined: | ||
a. Reference levels | |||
A key point for between the herds benchmarking process is how to compare with the appropriate benchmarking group and how to establish a target related to this group. For that reason, it is important to define a comparable reference level. Reference level could be defined by herd size, production level, geographic location, flooring and housing systems, season, parity, age and stage of lactation. | A key point for between the herds benchmarking process is how to compare with the appropriate benchmarking group and how to establish a target related to this group. For that reason, it is important to define a comparable reference level. Reference level could be defined by herd size, production level, geographic location, flooring and housing systems, season, parity, age and stage of lactation. | ||
b. Cows at risk | |||
One of the challenges of a benchmark calculation is the definition of the denominator. By definition it should be equal to the number of cows at risk in the time period. However, the concept of “cows at risk during the time period” may be inaccurate if not all cows are trimmed or checked. So, if we consider cows at risk as cows present in the herd at any moment of the time period that means that non-trimmed cows are assumed to be “healthy cows”. While if we consider cows at risk as trimmed cows during the time period, then the calculated rates depend on the percentage of trimmed cows. In situations of regular lameness screening (every 1-4 weeks) then this assumption may be valid. Detection may also be influenced by the timing of the foot inspection, with lesion detection rates higher at 60-120 days into lactation in most herds. The other critical point is that we deal with open herds where animals are leaving and entering the herd throughout the time period. Dohoo et al. (2009)<ref>Dohoo I., Martin W. and Stryhn H. 2009. Veterinary Epidemiologic research. 2nd Edition. Published by VER Inc. Canada. </ref> reported that animals for which there is a loss of follow-up during the time period are called withdrawals and the simplest way of dealing with them is to subtract half the number of withdrawals from the population at risk. However, calculating animal-days within the herd is perhaps the most precise way to account for withdrawals. | One of the challenges of a benchmark calculation is the definition of the denominator. By definition it should be equal to the number of cows at risk in the time period. However, the concept of “cows at risk during the time period” may be inaccurate if not all cows are trimmed or checked. So, if we consider cows at risk as cows present in the herd at any moment of the time period that means that non-trimmed cows are assumed to be “healthy cows”. While if we consider cows at risk as trimmed cows during the time period, then the calculated rates depend on the percentage of trimmed cows. In situations of regular lameness screening (every 1-4 weeks) then this assumption may be valid. Detection may also be influenced by the timing of the foot inspection, with lesion detection rates higher at 60-120 days into lactation in most herds. The other critical point is that we deal with open herds where animals are leaving and entering the herd throughout the time period. Dohoo et al. (2009)<ref>Dohoo I., Martin W. and Stryhn H. 2009. Veterinary Epidemiologic research. 2nd Edition. Published by VER Inc. Canada. </ref> reported that animals for which there is a loss of follow-up during the time period are called withdrawals and the simplest way of dealing with them is to subtract half the number of withdrawals from the population at risk. However, calculating animal-days within the herd is perhaps the most precise way to account for withdrawals. | ||
c. Time period at risk | |||
Benchmark calculation should be performed on a reference period of time which allows a fair comparison within and across herds with different management systems and at different times of the year. The time period could be defined as a year, season or lactation period. | Benchmark calculation should be performed on a reference period of time which allows a fair comparison within and across herds with different management systems and at different times of the year. The time period could be defined as a year, season or lactation period. | ||
| Line 3,668: | Line 3,668: | ||
* Insufficient monitoring that results in late detection of cows requiring additional care; | * Insufficient monitoring that results in late detection of cows requiring additional care; | ||
* Poor management, particularly of transition cows; | * Poor management, particularly of transition cows; | ||
* Insufficient body condition (<2; Randall ''et al''., 2015 <ref>Randall L. V., M. J. Green, M. G. G. Chagunda, C. Mason, S. C. Archer, L. E. Green, and J. N. Huxley. 2015. Low body condition predisposes cattle to lameness: An 8-year study of one dairy herd. J. Dairy Sci. 98:3766–3777.</ref>/ For reference, see the Section 5 of the ICAR Guidelines for conformation recording); | * Insufficient body condition (<2; Randall ''et al''., 2015 <ref>Randall L. V., M. J. Green, M. G. G. Chagunda, C. Mason, S. C. Archer, L. E. Green, and J. N. Huxley. 2015. Low body condition predisposes cattle to lameness: An 8-year study of one dairy herd. J. Dairy Sci. 98:3766–3777.</ref>/ For reference, see the [[Section 05 – Conformation Recording|Section 5]] of the ICAR Guidelines for conformation recording); | ||
* Parity; | * Parity; | ||
* Physical hazards. | * Physical hazards. | ||
| Line 4,215: | Line 4,215: | ||
</div> | </div> | ||
=last= | = Calving traits in Dairy Cattle = | ||
<div class="mw-collapsible mw-collapsed"> | |||
<div> | |||
== Introduction == | |||
The purpose of these ICAR guidelines for recording of calving performance traits in dairy cattle is to give recommendations on recording, data validation and use of information in herd management, documentation of animal welfare, benchmarking, and genetic evaluations. For beef breeds please see Section 3 of the ICAR guidelines for Beef Cattle Recording. | |||
== Definitions and terminology == | |||
The main calving traits are stillbirth and calving ease. Other relevant traits are calf size and gestation length. All these traits have both direct and maternal aspects. | |||
Stillbirth is one of the major issues related to the calving. Figures suggested that the frequency has increased in dairy herds, although the reasons are still not clear (Mee, 2020). Stillbirth is defined as a calving in which the calf is born dead or dies during the first 24 hours after parturition. Other terms like calf livability, perinatal survival, or calf mortality (alive or dead) are also used in addition or instead of stillbirth. In this document we use stillbirth. | |||
Calf mortality may be classified as abortion if it is stillborn before 260 days of gestation, and as stillbirth if it is after 260 days of gestation (Mee, 2020). Calf mortality later than 24 hours after parturition and mortality of young stock will not be considered further in this guideline. | |||
Calving ease is defined as how easy or difficult the calving was. In this document we use calving ease, other terms such as calving difficulty and dystocia are used for similar traits. | |||
Gestation length is the number of days between conception date (usually the last insemination date) and the calving date. Average dairy cattle gestation length is +/- 280 days. | |||
Calf size at birth (or calf birth weight). Often assessed as a subjective score. Calf size is associated with calving ease, stillbirth, and calf mortality. For Holstein the average calf is about 40 kg with a standard deviation of 4 to 5 kg. | |||
== Data recording == | |||
Registration of calving traits should be done for all calvings within all herds. Calving information is usually recorded by the dairy farmer. In some countries severe cases of dystocia may be recorded via veterinary treatments and be available from health recording system. | |||
=== Recording of calving traits === | |||
The most important traits to record are: Calving ease and stillbirth. | |||
Also recommended: Gestation length and calf size. | |||
==== Important information for calving traits recording ==== | |||
In general, the following information should be ensured for calving traits: | |||
* Herd ID | |||
* Cow ID | |||
* Parity/lactation number | |||
* Calving date | |||
* ID of calf/calves<sup>1</sup> | |||
* Sex of calf/calves<sup>2</sup> | |||
* Number of calves born at calving (twin information) | |||
* Sire ID | |||
* Sire breed | |||
* Calf from embryo? (yes/no); if yes, specify if from Ovum pick up (OPU) | |||
<sup>1</sup> ''ID of calf. From identification & registration perspective all live animals should be identified within 48 hours, but regulations regarding calves born dead may differ between countries. A “dummy” ID needs to be assigned to stillborn calves that have not been assigned an official ID.'' | |||
''<sup>2</sup> Sex of calf should always be recorded, as it has a strong influence on calving ease and the importance of including this in the evaluation model increases when sexed semen is used. This also includes the sex of stillborn calves.'' | |||
==== Other relevant information for calving traits recording ==== | |||
The following may be useful information related to calving traits: | |||
* Detailed information related to embryo transfer process (see: [[Section 06 – AI and ET Data and Fertility Analysis|Section 06]] of the ICAR guidelines for recording AI and ET and reporting fertility. | |||
* Calf size | |||
* Insemination dates are needed for calculation of gestation length | |||
* Pelvic area or rump width and rump angle | |||
* Information on sexed semen | |||
==== Calving Ease scoring scale ==== | |||
The calving ease score should describe how easy or difficult the calving was. The optimum would be to distinguish between the following situations: | |||
* Unassisted unobserved calving (if farmer not present) | |||
* Unassisted observed calving (no assistance needed) | |||
* Easy pull: calving which really needed some manual assistance | |||
* Hard pull: some mechanical assistance required | |||
* Difficult calving: vet assistance required. | |||
* Caesarean section | |||
* Embryotomy | |||
All details may not always be relevant or needed. We recommend that calving ease should be scored in 4 classes. The classes should be well defined and allow easy determination of the class to help keeping accurate records. | |||
<ol style="list-style-type: number;"> | |||
<li>'''Easy, unassisted:''' calving without any assistance (also if unobserved/farmer not present)</li> | |||
<li>'''Easy pull:''' calving which really needed some manual assistance</li> | |||
<li>'''Difficult calving/Hard pull''': some mechanical assistance required, with or without veterinarian aid</li> | |||
<li>'''Caesarean section/embryotomy'''</li> | |||
</ol> | |||
We recommend that caesarean section and embryotomy be recorded in a separate category, such that these records can easily be omitted when data are used for genetic evaluation. | |||
Other scaling systems exist, and the level of detail needed may vary between breeds and depend on the purpose of data use. | |||
==== Stillbirth scoring scale ==== | |||
Stillbirth is defined as a calving in which the calf is born dead or dies during the first 24 hours after parturition. We recommend scoring stillbirth using two classes: | |||
<ol style="list-style-type: decimal;"> | |||
<li>'''Alive'''</li> | |||
<li>'''Dead at birth or dead within the first 24 hours'''</li> | |||
</ol> | |||
Some countries record stillbirth using 3 categories: 1. Alive, 2=Dead at birth, 3=Alive at birth but dead within the first 24 hours. | |||
Calves alive at birth and passing the 24-hour threshold alive must be identified and recorded as such. Therefore, a calf born without information on calf identification and live status should not be assumed to be alive calf. | |||
==== Recording gestation length ==== | |||
Gestation length is computed from insemination date and calving date (number of days). | |||
==== Recording calf size ==== | |||
Calf size at birth is often assessed as a subjective score, e.g. small, medium, large. A more accurate alternative would be calf birth weight. | |||
=== Documentation and data flow === | |||
The farmer/dairy producer used to fill in the birth registration for each new born and delivered it to DHI /milk recording organisation. Information related to how the calving took place and on the status of liveability of each calf, was until recently filled in the same form but as optional information, in most countries. | |||
Nowadays, all information related to the calving is becoming more and more relevant, mainly for use in genetic evaluations. As soon as possible after each delivery, calving ease score should be set by the farmer and reported in connection with new born animal id registration, mainly through digital solutions, to assure a complete and an accurate data recording. Digital applications, widely used for animal registration, allowed by different drop-down-menu options recording all information about calving, such as the number of calves born, the sex of each new calf, the size of each new calf and its liveability. For herds without access to digital solutions, information could be recorded by DHI/milk recording technicians or by filling all the information in the traditional registration form and sent it to the correspondent registration organisation within each country. | |||
== Data validation == | |||
The main issues related with calving traits data recording are: | |||
* Potential under-reporting of dystocia cases: That may result in herds with very low frequency of some calving ease classes. | |||
* Potential misinterpretation of the scale: the differentiation between scores 1 and 2 may not always be well understood. That is why farmers should take into consideration the cow’s needs rather than what they did. For herds with more frequent assisted calving than unassisted calving, scores definition should be discussed with the farmer. | |||
The data validation process has to ensure the usefulness of this information for each purpose and avoid loss of information. | |||
Data validation is generally done in two steps called data verification and data editing. | |||
* '''Data verification''' | |||
Basic checks on format and completeness, at the incorporation of data. | |||
'''For example,''' Plausibility of ID: ''animal-ID, herd-ID, calving ease score''. Reasonableness of dates: ''date of insemination, date of calving.'' | |||
Checking the correctness of data depend on the purpose of use and on the information source. | |||
* '''Data editing''' | |||
Data editing should include a clear protocol that describes how to validate the quality of the data from each farm. For calving ease, a check on the distribution of classes is needed. If a herd has a high percentage of records in a single class, the calving ease records from that herd period should be checked with the farmer, and depending on the data uses, they might be omitted. | |||
To define the required period, we should bear in mind that we need to define a minimum number of calving. Depending on the use of the data a minimum frequency could be required. | |||
'''For genetic evaluation the following edits should be considered:''' | |||
* If frequency of a single class of calving ease is very low (Less than 1%) it should be combined with the neighbouring class or increased the period. If classes are combined due to the number of cases, data should continuously be carefully monitored. The limits here should follow local circumstances. | |||
* Exclude records of multiple births. | |||
* How to handle calving records resulting from embryo transfer (ET) is a question. | |||
** Exclude all ET records. | |||
** Modelling ET correctly: direct and maternal effects - dam of embryo and cow carrying the calf (recipient cow), pedigree and pe effects | |||
** Include method for ET. | |||
* Breed of sire of calf. How to handle beef on dairy | |||
** Exclude if sire or maternal grandsire of calf is unknown or of another breed. | |||
One solution to these issues is to edit the data used for genetic evaluation and exclude calving records resulting from embryo transfer, records from multiple births (twins), and if sire or maternal grandsire of calf is unknown or of another breed. | |||
'''For herd management and benchmarking the following edits should be considered:''' | |||
Data recorded about calving are valuable for herd management and decision-making process. For this use data should be as complete as possible and only records that are completely not consistent with other sources of information such as milk recording data, should be removed. | |||
For benchmarking use, the most important check should be made on the representativeness of the reference group at which belong each record. | |||
== Use of data == | |||
Routinely recorded calving performance is valuable information that can be used in herd management, documentation of animal welfare, benchmarking and for genetic evaluations. | |||
=== Genetic evaluation === | |||
'''Model''' | |||
Ideally, the categorical traits of stillbirth and calving ease should be analyzed using a multivariate threshold model with direct and maternal effects (e.g. Heringstad et al 2007<ref>Heringstad, B., Y.M. Chang, M. Svendsen, and D. Gianola. 2007. Genetic analysis of calving difficulty and stillbirth in Norwegian Red cows. Journal of Dairy Science 90: 3500-3507. <nowiki>https://doi.org/10.3168/jds.2006-792</nowiki></ref>; Cole et al., 2007<ref>Cole, J.B., G.R. Wiggans, and P.M. VanRaden. 2007. Genetic evaluation of stillbirth in United States Holsteins using a sire-maternal grandsire threshold model. J Dairy Sci. 90:2480-2488. <nowiki>https://doi.org/10.3168/jds.2006-435</nowiki></ref>). However, linear models may often be the model of choice for routine genetic evaluation as they are fast, easy to implement, and in most cases gives a very similar ranking of animals as more advanced models. Eaglen et al. (2012) <ref>Eaglen, S.A., M.P. Coffey, J.A. Woolliams, and E. Wall. 2012. Evaluating alternate models to estimate genetic parameters of calving traits in United Kingdom Holstein-Friesian dairy cattle. Genet. Sel. Evol. 44(1):23. doi: 10.1186/1297-9686-44-23</ref>compared models for calving traits and concluded that multi-trait models had an advantage over univariate models and that extended sire models (i.e. sire maternal grandsire model) are more practical and robust than animal models. | |||
The models used for genetic evaluation must include both direct and maternal effects for all calving traits. Direct effects are the calf’s genetic potential for being born easily and alive, while maternal effects are the cow’s genetic potential for easy calving and liveborn calves | |||
'''Traits and trait definitions''' | |||
Precorrection for heterogenous variance may be needed. EuroGenomics (2022) suggest that if a linear model approach is chosen, should approximation to normal distribution using e.g. Snell scores be used (Snell, 1964<ref>Snell, E. J. 1964. A Scaling Procedure for Ordered Categorical Data. Biometrics Vol. 20, No. 3 (Sep., 1964), pp. 592-607. <nowiki>https://doi.org/10.2307/2528498</nowiki></ref>). | |||
Calving ease is recorded as an ordered categorical trait. How many classes to be used in genetic evaluation is a question. If the frequency is low than 1% in any classes, it may be needed to combine with neighbouring class. However, if the frequency of any class is higher than 90%, the data of the herd-period of time should be eliminated when the aim is estimating breeding values. | |||
In some countries (USA for example) calving ease is defined as calving difficulty expressed as percentage of births of bull calves that are difficult in primiparous heifers and in adult cows. | |||
Calf size and gestation length are examples of genetically correlated traits that may be useful indicator traits to include in a multivariate model together with stillbirth and calving ease. | |||
If multiple parities are included in the genetic evaluation we recommend that first and later parities are treated as genetically correlated trait. Genetic correlations far from 1 suggest that first and later lactation should not be assumed to be the same trait across parities. | |||
'''Effects to consider''' | |||
Effects to consider in the model for genetic evaluation of calving traits, in addition to the standard effects such as the cow’s age, contemporary group, and parity, are the sex of calf(s) and the number of calves born (twin information). Calves coming from embryo transfer must be modelled correctly, as a direct effect is coming from the pedigree of the dam that provided the embryo, while the maternal effect (genetic and potentially permanent environment) is coming from the pedigree of the dam that carries the calf. | |||
Consider whether interaction terms to correct for environmental time trends are needed, such as Herd-Year-Age or Herd-Year-Month of calving. | |||
'''Proofs published''' | |||
The traits delivered to INTERBULL are only first parity calving traits. It would be an improvement if INTERBULL would allow sending BV predicted for multiple lactations. The traits considered are direct and maternal calving ease and direct and maternal stillbirth. For details related to national genetic evaluations of calving traits see: https://interbull.org/ib/geforms | |||
Calving ease direct: It indicates the influence of the sire on calving ease. | |||
Maternal calving ease: It indicates how easily a sire’s daughter will calve compared to the daughters of other sires. | |||
Breeding values for gestation length and calf size could be useful for herd management purposes. | |||
'''Genetic parameters''' | |||
'''''Heritability'''''. The heritabilities of calving performance traits are in general low. The range of heritabilities used for first parity calving traits in national genetic evaluations by countries that deliver calving traits to Interbull are in Table 29 (From: https://interbull.org/ib/geforms), and details are given in Appendix 3: heritability of calving traits used in national genetic evaluations. | |||
<center> | |||
{| class="wikitable" | |||
| colspan="5" style="text-align:center;" |'''''Table 30. Range of heritabilities of calving traits used in national genetic evaluations.''''' | |||
|- style="background-color:#efefef;" | |||
| | |||
| colspan="2" style="text-align:center;" |'''Calving Ease''' | |||
| colspan="2" style="text-align:center;" |'''Stillbirth''' | |||
|- | |||
| | |||
| style="text-align:center;" |Direct | |||
| style="text-align:center;" |Maternal | |||
| style="text-align:center;" |Direct | |||
| style="text-align:center;" |Maternal | |||
|- | |||
| style="text-align:left;" |Linear model | |||
| style="text-align:center;" |0.021 – 0.24 | |||
| style="text-align:center;" |0.023 – 0.158 | |||
| style="text-align:center;" |0.002 – 0.04 | |||
| style="text-align:center;" |0.010 – 0.086 | |||
|- | |||
| style="text-align:left;" |Threshold model | |||
| style="text-align:center;" |0.056 – 0.08 | |||
| style="text-align:center;" |0.027 - 0.067 | |||
| style="text-align:center;" |0.03 - 0.059 | |||
| style="text-align:center;" |0.058 - 0.066 | |||
|} | |||
</center> | |||
'''''Genetic correlations.''''' In routine genetic evaluations are the genetic correlation between direct and maternal calving traits often assumed to be zero (https://interbull.org/ib/geforms). Heringstad et al (2007)<ref>Heringstad, B., Y.M. Chang, M. Svendsen, and D. Gianola. 2007. Genetic analysis of calving difficulty and stillbirth in Norwegian Red cows. Journal of Dairy Science 90: 3500-3507. <nowiki>https://doi.org/10.3168/jds.2006-792</nowiki></ref> estimated strong genetic correlations between direct stillbirth and direct calving difficulty (0.79), and between maternal stillbirth and maternal calving difficulty (0.62) for Norwegian Red cows, whereas all genetic correlations between direct and maternal effects within or between traits were close to zero, suggesting that bulls should be evaluated both as sire of calf (direct effect) and sire of the cow (maternal effect). | |||
=== Herd management use === | |||
Information on calving traits are useful in herd management. Farmers try to consider an endless list of best practices and recommended standards to ensure a good preparation for calving. Nevertheless, there is no clear evidence of their effectiveness. On the other hand, it is known that herd management to reduce dystocia cases should start with heifers’ development. | |||
The best way to know if something is going wrong around calving within a specific farm is by using calving ease scores and monitoring the situation over different periods of time. Reducing the number of dystocia cases will improve cow- as well as calf health and animal welfare. Examples on measures that can improve calving performance: | |||
* Make breeding plans to avoid difficult calvings. Consider the bulls breeding value for calving ease and calf size (direct effect, sire of calf) when choosing which bulls to use for each cow. Avoid using bulls that gives large calves to heifers/small cows and to cows that had difficult calving in the past (e.g. GENEX, 2022<ref>GENEX. 2022. How much calving ease is enough? Available at <nowiki>https://genex.coop/how-much-calving-ease-is-enough/</nowiki></ref>). | |||
* Breeding values for gestation length (direct effect, sire of calf) can be used to predict expected calving date more accurately and thereby be an useful herd management tool. | |||
* Use information on calving performance when making culling decisions for the herd. | |||
Unfortunately, evidence-based best management practices for animals around calving are largely unknown, with several knowledge gaps still existing on the subject. Further investigations on the effect of management practices, on the effect of environmental conditions on calving time, and on cow-calving behaviours are needed to understand better calving process and help farmers with more information about how to improve dairy cow’s management around calving period. Meanwhile, analysing, throughout seasons/years of calving, the easy-calving-score frequencies to detect any issues and check all risk factors to find out their grounds. | |||
=== Animal welfare use === | |||
Ensuring a high animal welfare on dairy industry may rely on many factors, which could be related to herd management, farm facilities and animal abilities. The objective way to assess animal welfare should be related to animal performances. Calving performance traits, considered as health or reproductive aspects by animal welfare expert, are ones of the important performances taken account by animal welfare protocol assessments. Routinely recorded herd data, such as records on stillbirths and dystocia, can be used for documentation of animal welfare status (Haskell et al. 2019<ref>Haskell (2019). Mapping the global use of welfare indicators for dairy cows.<nowiki>https://www.icar.org/Documents/Prague-2019/Presentations/02%20-%20Marie%20Haskell.pdf</nowiki></ref>; OIE, 2020<ref>OIE. 2020: Terrestrial Animal Health Code. <nowiki>https://rr-europe.oie.int/wp-content/uploads/2020/08/oie-terrestrial-code-1_2019_en.pdf</nowiki>.</ref>). | |||
'''Acknowledgements''' | |||
We are grateful to EuroGenomics, who shared their knowledge and experience, and gave access to their document “Golden Standard for calving traits (https://www.eurogenomics.com/golden-standards.html), which aim at harmonization of traits within the EuroGenomics collaboration. | |||
== Appendix 3: Heritability of calving traits used in national genetic evaluations. == | |||
<center> | |||
{| class="wikitable" | |||
| colspan="7" style="text-align:left;" |'''''Table 1. Heritability of calving traits used in national genetic evaluations by countries that deliver calving traits to Interbull (from: https://interbull.org/ib/geforms, accessed March 2022).''''' | |||
|- style="background-color:#efefef;" | |||
| rowspan="2" |'''Country''' | |||
| rowspan="2" |'''Breed<sup>1</sup>''' | |||
| rowspan="2" |'''Model<sup>2</sup>''' | |||
| colspan="2" style="text-align:center;" |'''Calving Ease''' | |||
| colspan="2" style="text-align:center;" |'''Stillbirth''' | |||
|- style="background-color:#efefef;" | |||
| style="text-align:center;" |Direct | |||
| style="text-align:center;" |Maternal | |||
| style="text-align:center;" |Direct | |||
| style="text-align:center;" |Maternal | |||
|- | |||
| style="text-align:left;" |Australia | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.07 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Belgium | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |ST AM | |||
| style="text-align:center;" |0.077 | |||
| style="text-align:center;" |0.023 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| rowspan="3" style="text-align:left;" |Canada | |||
| style="text-align:center;" |HOL, BWS, GUE | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.036 | |||
| style="text-align:center;" |0.125 | |||
| style="text-align:center;" |0.0055 | |||
| style="text-align:center;" |0.071 | |||
|- | |||
| style="text-align:center;" |AYR | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.048 | |||
| style="text-align:center;" |0.086 | |||
| style="text-align:center;" |0.004 | |||
| style="text-align:center;" |0.06 | |||
|- | |||
| style="text-align:center;" |JER | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.021 | |||
| style="text-align:center;" |0.158 | |||
| style="text-align:center;" |0.0018 | |||
| style="text-align:center;" |0.0712 | |||
|- | |||
| rowspan="2" style="text-align:left;" | Denmark, Finland, Sweden | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.08 | |||
| style="text-align:center;" |0.06 | |||
| style="text-align:center;" |0.04 | |||
| style="text-align:center;" |0.035 | |||
|- | |||
| style="text-align:center;" |RDC | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.06 | |||
| style="text-align:center;" |0.04 | |||
| style="text-align:center;" |0.035 | |||
|0.02 | |||
|- | |||
| rowspan="2" style="text-align:left;" |France | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |THR | |||
| style="text-align:center;" |0.056 | |||
| style="text-align:center;" |0.032 | |||
| style="text-align:center;" |0.03 | |||
| style="text-align:center;" |0.066 | |||
|- | |||
| style="text-align:center;" |BSW | |||
| style="text-align:center;" |THR | |||
| style="text-align:center;" |0.074 | |||
| style="text-align:center;" |0.043 | |||
| style="text-align:center;" |0.059 | |||
| style="text-align:center;" |0.058 | |||
|- | |||
| style="text-align:left;" |Germany, Austria, Luxemburg | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.048 | |||
| style="text-align:center;" |0.039 | |||
| style="text-align:center;" |0.027 | |||
| style="text-align:center;" |0.054 | |||
|- | |||
| style="text-align:left;" |Austria, Germany | |||
| style="text-align:center;" |BSW | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.057 | |||
| style="text-align:center;" |0.065 | |||
| style="text-align:center;" |0.013 | |||
| style="text-align:center;" |0.010 | |||
|- | |||
| style="text-align:left;" |Austria, Germany, Czech Republic | |||
| style="text-align:center;" |FL | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.066 | |||
| style="text-align:center;" |0.105 | |||
| style="text-align:center;" |0.012 | |||
| style="text-align:center;" |0.011 | |||
|- | |||
| style="text-align:left;" |GBR | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |S-MGS | |||
| style="text-align:center;" |0.068 | |||
| style="text-align:center;" |0.044 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Hungary | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.24 | |||
| style="text-align:center;" |0.156 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Ireland | |||
| style="text-align:center;" |HOL, RDC | |||
| style="text-align:center;" |AM | |||
| style="text-align:center;" |0.09 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Israel | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.06 | |||
| style="text-align:center;" |0.03 | |||
| style="text-align:center;" |0.03 | |||
| style="text-align:center;" |0.014 | |||
|- | |||
| style="text-align:left;" |Italia | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |THR | |||
| style="text-align:center;" |0.08 | |||
| style="text-align:center;" |0.036 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Netherlands | |||
| style="text-align:center;" |All | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.068 | |||
| style="text-align:center;" |0.048 | |||
| style="text-align:center;" |0.038 | |||
| style="text-align:center;" |0.086 | |||
|- | |||
| style="text-align:left;" |New Zeeland | |||
| style="text-align:center;" |All | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.045 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Norway | |||
| style="text-align:center;" |RDC | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.068 | |||
| style="text-align:center;" |0.048 | |||
| style="text-align:center;" |0.011 | |||
| style="text-align:center;" |0.011 | |||
|- | |||
| style="text-align:left;" |Poland | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |MT AM | |||
| style="text-align:center;" |0.048 | |||
| style="text-align:center;" |0.039 | |||
| style="text-align:center;" |0.027 | |||
| style="text-align:center;" |0.054 | |||
|- | |||
| style="text-align:left;" |Slovakia | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |THR, S-MGS | |||
| style="text-align:center;" |0.072 | |||
| style="text-align:center;" |0.067 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Spain | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |THR, S-MGS | |||
| style="text-align:center;" |0.072 | |||
| style="text-align:center;" |0.027 | |||
| style="text-align:center;" | | |||
| style="text-align:center;" | | |||
|- | |||
| style="text-align:left;" |Switzerland | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |MT, S-MGS | |||
| style="text-align:center;" |0.053 | |||
| style="text-align:center;" |0.041 | |||
| style="text-align:center;" |0.007 | |||
| style="text-align:center;" |0.02 | |||
|- | |||
| style="text-align:left;" |USA | |||
| style="text-align:center;" |HOL | |||
| style="text-align:center;" |THR, S-MGS | |||
| style="text-align:center;" |0.072 | |||
| style="text-align:center;" |0.053 | |||
| style="text-align:center;" |0.03 | |||
| style="text-align:center;" |0.065 | |||
|} | |||
</center> | |||
<sup>1</sup>Breed: HOL=Holstein, RDC=Red Dairy Cattle, AYR=Ayrshire, JER=Jersey; FL=Fleckvieh. | |||
<sup>2</sup>MT=multi-trait model, AM=animal model, S-MGS=Sire maternal grandsire, THR=Threshold model. | |||
</div> | |||
</div> | |||
Latest revision as of 09:42, 10 September 2024
Dairy Cattle Health
Technical abstract
Improved health of dairy cattle is of increasing economic importance. Poor health results in greater production costs through higher veterinary bills, additional labour costs, and reduced productivity. Animal welfare is also of increasing interest to both consumers and regulatory agencies because healthy animals are needed to provide high-quality food for human consumption. Furthermore, this is consistent with the European Union animal health strategy that emphasizes disease prevention over treatment. Animal health issues may be addressed either directly, by measuring and selecting against liability to disease, or indirectly by selecting against traits correlated with injury and illness. Direct observations of health and disease events, and their inclusion in recording, evaluation and selection schemes, will maximize the efficiency of genetic selection programs. The Scandinavian countries have been routinely collecting and utilizing those data for years, demonstrating the feasibility of such programs. Experience with direct health data in non-Scandinavian countries is still limited. Due to the complexity of health and diseases, programs may differ between countries. This document presents best-practices with respect to data collection practices, trait definition, and use of health data in genetic evaluation programs and can be extended to its use for other farm management purposes.
Introduction
The improvement of cattle health is of increasing economic importance for several reasons. Impaired health results in increased production costs (veterinary medical care and therapy, additional labour, and reduced performance), while prices for dairy products and meat are decreasing. Consumers also want to see improvements in food safety and better animal welfare. Improvement in the general health of the cattle population is necessary for the production of high-quality food and implies significant progress with regard to animal welfare. Improved welfare also is consistent with the EU animal health strategy, which states that that prevention is better than treatment (European Commission, 2007[1]).
Health issues may be addressed either directly or indirectly. Indirect measures of health and disease have been included in routine performance tests by many countries. However, directly observed measures of health and disease need to be included in recording, evaluation and selection schemes in order to increase the efficiency of genetic improvement programs for animal health.
In the Scandinavian countries, direct health data have been routinely collected and utilized for years, with recording based on veterinary medical diagnoses (Nielsen, 2000[2]; Philipsson & Linde, 2003[3]; Østerås & Sølverød, 2005[4]; Aamand, 2006[5]; Heringstad et al., 2007[6]). In the non-Scandinavian countries experience with direct health data is still limited, but interest in using recorded diagnoses or observations of disease has increased considerably in recent years (Zwald et al., 2006a[7],b[8]; Neuenschwander et al., 2008[9]; Neuenschwender, 2010[10]; Appuhamy et al., 2009[11]; Egger-Danner et al., 2010[12], Egger-Danner et al., 2012[13], Koeck et al., 2012a[14],b[15], Neuschwander et al., 2012[16]).
Due to the complex biology of health and disease, guidelines should mainly address general aspects of working with direct health data. Specific issues for the major disease complexes are discussed, but breed- or population-specific focuses may require amendments to these guidelines.
Types and sources of data
Types of data
The collection of direct information on health and disease status of individual animals is preferable to collection of indirect information. However, population-wide collection of reliable health information may be easier to implement for indirect rather than direct measures of health. Analyses of health traits will probably benefit from combined use of direct and indirect health data, but clear distinctions must be drawn between these two types of data:
Direct health information
- Diagnoses or observations of diseases
- Clinical signs or findings indicative of diseases
Indirect health information
- Objectively measurable indicator traits (e.g., somatic cell count, milk urea nitrogen, health biomarkers)
- Subjectively assessable indicator traits (e.g., body condition score, conformation scores)
Health data may originate from different data sources which differ considerably with respect to information content and specificity. Therefore, the data source must be clearly indicated whenever information on health and disease status is collected and analysed. When data from different sources are combined, the origin of data must be taken into account when defining health traits.
In the following sections, possible sources of health data are discussed, together with information on which types of data may be provided, specific advantages and disadvantages associated with those sources, and issues which need to be addressed when using those sources.
Sources of data
Veterinarians
Content
- Primarily report direct health data.
- Provide disease diagnoses (documented reasons for application of pharmaceuticals), possibly supplemented by findings indicative of disease, and/or information on indicator traits.
Advantage
- Information on a broad spectrum of health traits.
- Specific veterinary medical diagnoses (high-quality data).
- Legal obligations of documentation in some countries (possible utilization of already established recording practices).
Disadvantages
- Only severe cases of disease may be reported (need for veterinary intervention and pharmaceutical therapy).
- Possible delay in reporting (gap between onset of disease and veterinary visit).
- Extra time and effort for recording (complete and consistent documentation cannot be taken for granted, recording routine and data flow need to be established).
Producers
Content
- Primarily direct health data.
- Disease observations ('diagnoses'), possibly supplemented by findings indicative of disease and/or information on indicator traits.
Advantages
- Information on a broad spectrum of health traits.
- Minor cases not requiring veterinary intervention may be included.
- First-hand information on onset of disease.
- Possible use of already-established data flow (routine performance testing, reporting of calving, documentation of inseminations).
Disadvantages
- Risk of false diagnoses and misinterpretation of findings indicative of disease (lack of veterinary medical knowledge).
- Possible need to confine recording to the most relevant diseases (modest risk of misinterpretation, limited extra time and effort for recording).
- Extra documentation might be needed.
- Need for expert support and training (veterinarian) to ensure data quality.
- Completeness of recording may vary, and may be dependent on work peaks on the farm.
Remarks
- Data logistics depend on technical equipment on the farm (documentation using herd management software (e.g. including tools to record hoof trimming, diseases, vaccinations,..), handheld for online recording, information transfer through personnel from milk recording agencies.
- Possible producer-specific documentation focuses must be considered in all stages of analyses (checks for completeness of health / disease incident documentation; see Kelton et al., 1998[17]).
- Preliminary research suggests that epidemiological measures calculated from producer-recorded data are similar to those reported in the veterinary literature (Cole et al., 2006[18]).
Expert groups (claw trimmer, nutritionist, etc.)
Content
- Direct and indirect health data with a spectrum of traits according to area of expertise.
Advantages
- Specific and detailed information on a range of health traits important for the producer (high-quality data),
- Possible access to screening data (information on the whole herd at a given point in time),
- Personal interest in documentation (possible utilization of already-established recording practices)
Disadvantages
- Limited spectrum of traits,
- Dependence on the level of expert knowledge (certification/licensure of recording persons may be advisable),
- Extra time and effort for recording (complete and consistent documentation cannot be taken for granted, recording routine and data flow need to be established)
- Business interests may interfere with objective documentation
Others (laboratories, on-farm technical equipment, etc.)
Content
- Indirect health data with spectrum of traits according to sampling protocols and testing requests, e.g., microbiological testing, metabolite analyses, hormone tests, virus/bacteria DNA, infrared-based measurements (Soyeurt et al., 2009a[19],b[20]).
Advantages
- Specific information on a range of health traits important for the producer (high quality data).
- Objective measurements.
- Automated or semi-automated recording systems (possible utilization of already established data logistics).
Disadvantages
- Interpretation with regard to disease relevance not always clear.
- Validation and combined use of data may be problematic.
| Table 1. Overview of the possible sources of direct and indirect health information. | ||
| Source of data | Type of data | |
| Direct health information | Indirect health information | |
| Veterinarian | Yes | Possibly |
| Producer | Yes | Possibly |
| Expert groups | Yes | Possibly |
| Others | No | Yes |
Data security
Data security is a universally important issue when collecting and using field data. However, the central role of dairy cattle health in the context of animal welfare and consumer protection implies that farmers and veterinarians are obligated to maintain high-quality records, emphasizing the particular sensitivity of health data.
The legal framework for use of health data has to be considered according to national requirements and applicable data privacy standards. The owner of the farm on which the data are recorded is the owner of the data and must enter into formal agreements before data are collected, transferred, or analysed. The following issues must be addressed with respect to data exchange agreements:
- Type of information to be stored in the health database, e.g., inclusion of details on therapy with pharmaceuticals, doses and medication intervals).
- Institutions authorized to administer the health database, and to analyse the data.
- Access rights of (original) health data and results from analyses of the data.
- Ownership of the data and authority to permit transfer and use of those data.
Enrolment forms for recording and use of health data (to be signed by the farmers) have been compiled by the institutions responsible for data storage and analysis or governmental authorities (e.g., Austrian Ministry of Health, 2010).
For any health database it must be guaranteed that:
- The individual farmers can only access detailed information on their own farm, and for animals only pertaining to their presence on that farm.
- The right to edit health data are limited.
- Access to any treatment information is confined to the farmer and the veterinarian responsible for the specific treatment, with the option of anonymizing the veterinary data.
Data security is a necessary precondition for farmers to develop enough trust in the system to provide data. The recording of treatment data is much more sensitive than only diagnoses, and the need to collect and store such data should be very carefully considered.
Documentation
Minimum requirements for documentation:
- Unique animal ID (ISO number).
- Place of recording (unique ID of farm/herd).
- Source of data (veterinarian, producer, expert group, others).
- Date of health incident.
- Type of health incident (standardized code for recording).
Useful additional documentation:
- Individual identification of the recording person.
- Details on respective health incident (exact location, severity).
- Type of recording and method of data transfer (software used for on-farm recording, online-transmission).
- Information on type of diagnosis (first or subsequent).
The systematic use and appropriate interpretation of direct and indirect health data requires that information on health status be combined with other information on the affected animals (basic information such as date of birth, sex, breed, sire and dam, farm/herd; calving dates, and performance records). Therefore, unique identification of the individual animals used for the health data base must be consistent with the animal ID used in existing databases.
Widespread collection of health data may benefit from legal frameworks for documentation and use of diagnostic data. European legislation requests documentation of health incidents which involved application of pharmaceuticals to animals in the food chain. Veterinary medical diagnoses may, therefore, be available through the treatment records kept by veterinarians and farmers. However, it must be ensured that minimum requirements for data recording are followed; in particular, it must be noted that animal identification schemes are not uniform within or across countries. Furthermore, it must be a clear distinction made between prophylactic and therapeutic use of pharmaceuticals, with the former being excluded from disease statistics. Information on prophylaxis measures may be relevant for interpretation of health data (e.g., dry cow therapy), but should not be misinterpreted as indicators of disease. While recording of the use of pharmaceuticals is encouraged it is not uniformly required internationally, and health data should be collected regardless of the availability of treatment information.
Standardization of recording
In order to avoid misinterpretation of health information and facilitate analysis, a unique code should be used for recording each type of health incident. This code must fulfil the following conditions:
- Clear definitions of the health incidents to be recorded, without opportunities for different interpretations.
- Includes a broad spectrum of diseases and health incidents, covering all organ systems, and address infectious and non-infectious diseases.
- Understandable by all parties likely to be involved in data recording.
- Permit the recording of different levels of detail, ranging from very specific diagnoses of veterinarian compared to very general diagnoses or observations by producers.
Starting from a very detailed code of diagnoses, recording systems may be developed that use only a subset of the more extensive code. However, the identical event identifiers submitted to the health database must always have the same meaning. Therefore, data must be coded using a uniform national, or preferably international, scheme before entering information into the central health database. In the case of electronic recording of health data, it is the responsibility of the software providers to ensure that the standard interface for direct and/or indirect health data is properly implemented in their products. When farmers are permitted to define their own codes the mapping of those custom codes to standard codes is a substantial challenge, and careful consideration should be paid to that problem (see, e.g., Zwald et al., 2004a[21]).
A comprehensive code of diagnoses with about 1,000 individual input options (diagnoses) is provided as an appendix to these guidelines. It is based on the code of diagnoses developed in Germany by the veterinarian Staufenbiel ('zentraler Diagnoseschlüssel') (Annex). The structure of this code is hierarchical, and it may represent a 'gold standard' for the recording of direct health data. It includes very specific diagnoses which may be valuable for making management decisions on farms, as well as broad diagnoses with little specificity for analyses which require information on large numbers of animals (e.g. genetic evaluation). Furthermore, it allows the recording of selected prophylactic and biotechnological measures which may be relevant for interpretation of recorded health data.
In the Scandinavian countries and in Austria codes with 60 to 100 diagnoses are used, allowing documentation of the most important health problems of cattle. Diagnoses are grouped by disease complexes and are used for documentation by treating veterinarians (Osteras et al., 2007[22]; Austrian Ministry of Health, 2010; Osteras, 2012[23]).
For documentation of direct health data by expert groups, special subsets of the comprehensive code may be used. Examples for claw trimmers can be found in the literature (e.g. Capion et al., 2008[24]; Thomsen et al.,2008[25]; Maier, 2009a, b[26]; Buch et al., 2011[27]).
When working with producer-recorded data, a simplified code of diagnoses should be provided which includes only a subset of the extensive code (Neuenschwander et al., 2008[28]; USDA, 2010[29]). Diagnoses included must be clearly defined and observable without veterinary medical expertise. Such a reduced code may, for example, consider mastitis, lameness, cystic ovarian disease, displaced abomasum, ketosis, metritis/uterine disease, milk fever and retained placenta (Neuenschwander et al., 2008[30]). The United States model (USDA, 2010[31]) is event-based, and permits very general reports (e.g., This cow had ketosis on this day."), as well as very specific ones (e.g., "This cow had Staph. aureus mastitis in the right, rear quarter on this day.").
Data quality
General quality checks
Mandatory information will be used for basic plausibility checks. Additional information can be used for more sophisticated and refined validation of health data when those data are available.
- The recording farm must be registered to record and transmit health data.
- If information on the person recording the data are provided, that individual must be authorized to submit data for this specific farm.
- The animal for which health information is submitted must be registered to the respective farm at the time of the reported health incident.
- The date of the health incident must refer to a living animal (must occur between the birth and culling dates), and may not be in the future.
- A particular health event can only be recorded once per animal per day.
- The contents of the transmitted health record must include a valid disease code. In the case of known selective recording of health events (e.g., only claw diseases, only mastitis, no calf diseases), the health record must fit the specified disease category for which health data are supposed to be submitted.
- For sources of data with limited authorization to submit health data, the health record must fit the specified disease category (e.g., locomotory diseases for claw trimmers, metabolic disorders for nutritionists).
Specific quality checks
In order to produce reliable and meaningful statistics on the health status in the cattle population, recording of health events should be as complete as possible on all farms participating in the health improvement program. Ideally, the intensity of observation and completeness of documentation should be the same for all animals regardless of sex, age, and individual performance. Only then will a complete picture of the overall health status in the population emerge. However, this ideal situation of uniform, complete, and continuous recording may rarely be achieved, so methods must be developed to distinguish between farms with desirably good health status of animals and farms with poor recording practices.
Countries with on-going programs of recording and evaluation of health data require a minimum number of diagnoses per cow and year (e.g., Denmark: 0.3 diagnoses; Austria: 0.1 first diagnoses); continuity of data registration needs to be considered. Farms that fail to achieve these values are automatically excluded from further analyses until their recording has improved. However, herd sizes need to be considered when defining minimum reporting frequencies to avoid possible biases in favour of larger or smaller farms. Any fixed procedure involves the risk of excluding farms with extraordinary good herd health, but to avoid biased statistics there seems to be no alternative to criteria for inclusion, and setting minimum lower limits for reporting. Different criteria will be needed for diseases that occur with low frequency versus those with high frequency, particularly when the cost of a rare illness is very high compared to a common one.
Because recording practices and completeness on farms may not be uniform across disease categories (e.g., no documentation of claw diseases by the producer), data should be periodically checked by disease category to determine what data should be included. Use of the most-thoroughly documented group of health traits to make decisions about inclusion or exclusion of a specific farm may lead to considerable misinterpretation of health data.
There are limited options to routinely check health data for consistency on a per animal basis. Some diagnoses may only be possible in animals of specific sex, age, or physiological state. Examples can be found in the literature (Kelton et al., 1998[32]; Austrian Ministry of Health, 2010). Criteria for plausibility checks will be discussed in the trait-specific part of these guidelines.
Keys to long-term success
Regardless of the sources of health data included, long-term acceptance of the health recording system and success of the health improvement program will rely on the sustained motivation of all parties involved. To achieve this, frequent, honest, and open communications between the institutions responsible for storage and analysis of health data and people in the field is necessary. Producers, veterinarians and experts will only adopt and endorse new approaches and technologies when convinced that they will have positive impacts on their own businesses. Mutual benefits from information exchange and favourable cost-benefit ratios need to be communicated clearly.
When a key objective of data collection is the development a of genetic improvement program for health, producers must be presented with a reasonable timeline for events. When working with low-heritability traits that are differentially recorded much more data will be necessary for the calculation of accurate breeding values than for typical production traits. It is very important that everyone is aware of the need to accumulate a sufficient dataset to support those calculations, which may take several years. This will help ensure that participants remain motivated, rather than become discouraged when new products are not immediately provided. The development of intermediate products, such as reports of national incidence rates and changes over time, could provide tools useful to producers between the start of data collection and the introduction of genetic evaluations.
Health reports, produced for each of the participating farms and distributed to authorized persons, will help to provide early rewards to those participating in health data recording. To assist with management decisions on individual farms, health reports should contain within-herd statistics (health status of all animals on the farm and stratified by age and/or performance group), as well as across-herd statistics based on regional farms of similar size and structure. Possible access to the health reports by authorized veterinarians or experts will help to maximize the benefits of data recording by ensuring that competent help with data interpretation is provided.
Trait definition
Most health incidents in dairy herds fit into a few major disease complexes (e.g., Heringstad et al., 2007[33]; Koeck et al., 2010a[34],b[35], Wolff, 2012[36]), each of which implies that specific issues be addressed when working with related health information. In particular, variation exists with regard to options for plausibility checks of incoming data including eligible animal group, time frame of diagnoses, and possibility of repeated diagnoses.
Distinctions must be drawn between diseases which may only occur once in an animal's lifetime (maximum of one record per animal) or once in a predefined time period (e.g., maximum of one record per lactation) on the one hand and disease which may occur repeatedly throughout the life-cycle. Assumptions regarding disease intervals, i.e., the minimum time period after which the same health incident may be considered as a recurrent case rather than an indicator of prolonged disease, need to be considered when comparing figures of disease prevalences and distributions. Furthermore, it must be decided if only first diagnoses or first and recurrent diagnoses are included in lifetime and/or lactation statistics. Differences will have considerable impact on comparability of results from health data analyses.
Udder health
Mastitis is the qualitatively and quantitatively most important udder health trait in dairy cattle (e.g. Amand et al., 2006[37]; Heringstad et al., 2007[38], Wolff, 2012[39]). The term mastitis refers to any inflammation of the mammary gland, i.e., to both subclinical and clinical mastitis. However, when collecting direct health data one should clearly distinguish between clinical and subclinical cases of mastitis. Subclinical mastitis is characterized by an increased number of somatic cells in the milk without accompanying signs of disease, and somatic cell count (SCC) has been included in routine performance testing by many countries, representing an indicator trait for udder health (indirect health data).
Cows affected by clinical mastitis show signs of disease of different severity, with local findings at the udder and/or perceivable changes of milk secretion possibly being accompanied by poor general condition. Recording of clinical mastitis (direct health data) will usually require specific monitoring, because reliable methods for automated recording have not yet been developed. Documentation should not be confined to cows in first lactation but include cows of second and subsequent lactations. Optional information on cases that may be documented and used for specific analyses includes
- Type of clinical disease (acute, chronic).
- Type of secretion changes (catarrhal, hemorrhagic, purulent, necrotizing).
- Evidence of pathogens which may be responsible for the inflammation.
- Location of disease (affected quarter or quarters).
- Presence of general signs of disease.
Appropriate analyses of information on clinical mastitis require consideration of the time of onset or first diagnosis of disease (days in milk). Clinical mastitis developing early and late in lactation may be considered as separate traits.
| Table 2. Udder health trait considerations. | ||
| Parameters to check incoming health data | Recommended inclusion criterion | Remarks |
| Eligible animal group | Heifers and cows (obligatory: sex = female) |
Exceptions possible (where appropriate, diagnoses in younger females may be considered separately) |
| Time frame of diagnoses | 10 days before calving to 305 days in milk | Exceptions possible (where appropriate, diagnoses beyond -10 to 305 days in milk may be considered separately; shorter reference periods may be defined) |
| Repeated diagnoses | Possible per animal and lactation (possibility of multiple diagnoses per lactation) |
Definition of minimum time period after which same diagnosis may be considered as recurrent case rather than prolonged disease |
Reproductive disorders
Reproductive disorders represents a set of diseases which have the same effect (reduced fertility or reproductive performance), but differ in pathogenesis, course of disease, organs involved, possible therapeutic approaches, etc. To allow the use of collected health data for improvement of management on the herd and/or animal level, recording of reproductive disorders should be as specific as possible.
Grouping of health incidents belonging to this disease complex may be based on the time of occurrence and/or organ involved. Within each of these disease groups, specific plausibility checks must be applied considering, for example, time frame of diagnoses and possibility of multiple diagnoses per lactation (recurrence). Fixed dates to be considered include the length of the bovine ovarian cycle (21 days) and the physiological recovery time of reproductive organs after calving (total length of puerperium: 42 days).
Gestation disorders and peri-partum disorders
Examples:
- Embryonic death, abortion.
- Bradytocia (uterine inertia), perineal rupture.
- Retained placenta, puerperal disease, ... .
Irregular oestrus cycle and sterility
Examples:
- Cystic ovaries, silent heat.
- Metritis (uterine infection), ...
| Table 3. Reproduction trait considerations. | ||
| Parameters to check incoming health data | Recommended inclusion criterion | Remarks |
| Eligible animal group | Heifers and cows | Minimum age should be consistent with performance data analyses |
| Time frame of diagnoses | Depending on type of disease | Fixed patho-physiological time frames should be considered (e.g. Duration of puerperium, cycle length) |
| Repeated diagnoses | Depending on type of disease: maximum of one diagnosis per animal (e.g. Genital malformation), maximum of one diagnosis per lactation (e.g. Retained placenta) or possibility of multiple diagnoses per lactation (e.g. Cystic ovaries) | Definition of minimum time period after which same diagnosis may be considered as recurrent case rather than prolonged disease (e.g. 21 days for cystic ovaries because of direct relation to the ovary cycle) |
Locomotory diseases
Recording of locomotory diseases may be performed on different level of specificity. Minimum requirement for recording may be documentation of locomotion score (lameness score) without details on the exact diagnoses. However, use of some general trait lameness will be of little value for deriving management measures.
Because of the heterogeneous pathogenesis of locomotory disease, recording of diagnoses should be as specific as possible.
Rough distinction may be drawn between claw diseases and other locomotory diseases, but results of health data analyses will be more meaningful when more detailed information is available. Therefore, recording of specific diagnoses is strongly recommended. Determination of the cause of disease and options for treatment and prevention will benefit from detailed documentation of affected structure(s), exact location, type and extent of visible changes. Such details may be primarily available through veterinarians (more severe cases of locomotory diseases) and claw trimmers (screening data and less severe cases of locomotory diseases). However, experienced farmers may also provide valuable information on health of limbs and claws.
Care must be taken when referring to terms from farmers' jargon, because definitions are often rather vague and diagnoses of diseases may be inconsistent. Documentation practices differ based on training and professional standards, e.g., claw trimmers and veterinarians, as well as nationally and internationally, and different schemes have been implemented in various on-farm data collection systems. To ensure uniform central storage and analysis of data, tools for mapping data to a consistent set of keys must to be developed, and unambiguous technical terms (veterinary medical diagnoses) should be used in documentation whenever possible.
Claw diseases
Examples:
- Laminitis complex (white line disease, sole haemorrhage, sole duplication, wall lesions, wall buckling, wall concavity).
- Sole ulcer (sole ulcer at typical site = rusterholz's disease, sole ulcer at atypical site, sole ulcer at tip of claw).
- Digital dermatitis (mortellaro's disease = hairy foot warts = heel warts = papillomatous digital dermatitis).
- Heel horn erosion (erosio ungulae = slurry heel).
- Interdigital dermatitis, interdigital phlegmon (interdigital necrobacillosis = foot rot), interdigital hyperplasia (interdigital fibroma = limax = tylom).
- Circumscribed aseptic pododermatitis, septic pododermatitis.
- Horn cleft, ... .
The expertise of professional claw trimmers should be used when recording claw diseases. In herds with regular claw trimming (by the producer or a professional claw trimmer) accessibility of screening data, i.e., information on claw status of all animals regardless of regular or irregular locomotion (lameness) or absence or presence of other signs of disease (e.g., swelling, heat), will significantly increase the total amount of available direct health data, enhancing the reliability of analyses of those traits. Incidences of claw diseases may be biased if they are collected on based on examinations, or treatment, of lame animals.
Other information about claws which may be relevant to interpret overall claw health status of the individual animal, such as claw angles, claw shape or horn hardness, also may be documented. Some aspects of claw conformation may already be assessed in the course of conformation evaluation. Analyses of claw disease may benefit from inclusion of such indirect health data.
Foot and claw disorders - Harmonized description
Refer to ICAR Claw Atlas for detailed descriptions. The Claw Atlas is available on the ICAR website:
- As a .pdf file in English here.
- Translations in twenty other languages here.
- As a poster in English here.
- As a poster in German here.
Other locomotory diseases
Examples:
- Lameness (lameness score).
- Joint diseases (arthritis, arthrosis, luxation).
- Disease of muscles and tendons (myositis, tendinitis, tendovaginitis).
- Neural diseases (neuritis, paralysis), ... .
Low frequencies of distinct diagnoses will probably interfere with analyses of other locomotory diseases involving a high level of specificity. Nevertheless, the improvement of locomotory health on the animal and/or farm level will require detailed disease information indicating causative factors which need to be eliminated. The use of data from veterinarians may allow deeper insight into improvement options. Despite a substantial loss of precision, simple recording of lame animals by the producers may be the easiest system to implement on a routine basis. Rapidly increasing amounts of data may then argue for including lameness or lameness score in advanced analyses.
| Table 4. Considerations for locomotion traits. | ||
| Parameters to check incoming health data | Recommended inclusion criterion | Remarks |
| Eligible animal group | No sex or age restriction | Sex- and/or age-dependent differences in intensity of systematic recording should be considered |
| Time frame of diagnoses | No time restriction | - |
| Repeated diagnoses | Possibility of multiple diagnoses per animal independent of lactation | Definition of minimum time period after which same diagnosis may be considered as recurrent case rather than prolonged disease (no clear physiological reference period) |
Metabolic and digestive disorders
The range of bovine metabolic and digestive disorders is generally rather broad, including diverse infectious and non-infectious disease. Although each of these diseases may have significant impacts on individual animal performance and welfare, few of them are of quantitative importance. Major diseases can broadly be characterized as disturbances of mineral or carbohydrate metabolism, which are caused in the lactating cow primarily by imbalances between dietary requirements and intakes.
Metabolic disorders
Examples:
- Milk fever (i.e., hypocalcaemia, periparturient paresis), tetany (i.e., hypomagnesiaemia).
- Ketosis (i.e., acetonaemia), ...
Digestive disorders
Examples:
- Ruminal acidosis, ruminal alkalosis, ruminal tympany.
- Abomasal tympany, abomasal ulcer, abomasal displacement (left displacement of the abomasum, right displacement of the abomasum).
- Enteritis (catarrhous enteritis, hemorrhagic enteritis, pseudomembranous enteritis, necrotisizing enteritis).
| Table 5. Considerations for metabolic traits. | ||
| Parameters to check incoming health data | Recommended inclusion criterion | Remarks |
| Eligible animal group | Depending on type of disease: no sex or age restriction or restriction to adult females (calving-related disorders) | Sex- and/or age-dependent differences in intensity of systematic recording should be considered |
| Time frame of diagnoses | Depending on type of disease: no time restriction or restriction to (extended) peripartum period | Possible definition of risk periods (where appropriate, diagnoses beyond may be considered separately) |
| Repeated diagnoses | Depending on type of disease: maximum of one diagnosis per lactation (e.g. Milk fever), possibility of multiple diagnoses per lactation and independent of lactation (e.g. Enteritis) | Definition of minimum time period after which same diagnosis may be considered as recurrent case rather than prolonged disease (no clear physiological reference period) |
Others diseases
Diseases affecting other organ systems may occur infrequently. However, recording of those diseases is strongly recommended to get complete information on the health status of individual animals. Interpretation of the effect of certain diseases on overall health and performance will only be possible, if the whole spectrum of health problems is included in the recording program.
Examples:
- Diseases of the urinary tract (hemoglobinuria, hematuria, renal failure, pyelonephritis, urolithiasis, ...).
- Respiratory disease (tracheitis, bronchitis, bronchopneumonia, ...).
- Skin diseases (parakeratosis, furunculosis, ...).
- Cardiovascular disease (cardiac insufficiency, endocarditis, myocarditis, thrombophlebitis, ...).
| Table 6. Considerations for other disease traits. | ||
| Parameters to check incoming health data | Recommended inclusion criterion | Remarks |
| Eligible animal group | No sex or age restriction | Sex- and/or age-dependent differences in intensity of systematic recording should be considered |
| Time frame diagnoses | No time restriction | - |
| Repeated diagnoses | Possibility of multiple diagnoses per animal independent of lactation (e.g. Tracheitis) | Definition of minimum time period after which same diagnosis may be considered as recurrent case rather than prolonged disease (no clear physiological reference period) |
Calf diseases
Impaired calf health may have considerable impact on dairy cattle productivity. Optimization of raising conditions will not only have short-term positive effects with lower frequencies of diseased calves, but also may result in better condition of replacement heifers and cows. However, management practices with regard to the male and female calves usually differ between farms and need to be considered when analysing health data. On most dairy farms the incentive to record health events systematically and completely will be much higher for female than for male calves. Therefore, it may be necessary to generally exclude the male calves from prevalence statistics and further analyses.
Examples:
- Omphalitis (omphalophlebitis, omphaloarteriitis, omphalourachitis).
- Umbilical hernia.
- Congenital heart defect (persitent ductus arteriosus botalli, patent foramen ovale, ...).
- Neonatal asphyxia.
- Enzootic pneumonia of calves.
- Disturbance of oesophageal groove reflex.
- Calf diarrhea, ... .
| Table 7. Considerations for calf health traits. | ||
| Parameters to check incoming health data | Recommended inclusion criterion | Remarks |
| Eligible animal group | Calves | Sex-dependent differences in intensity of systematic recording should be considered |
| Time frame of diagnoses | Depending on type of disease (e.g. Neonatal period, suckling period) | Possible definition of risk periods (where appropriate, diagnoses beyond may be considered separately) |
| Repeated diagnoses | Depending on type of disease: maximum of one diagnosis per animal (e.g. Neonatal asphyxia) or possibility of multiple diagnoses per animal (e.g. Diarrhea) |
Definition of minimum time period after which same diagnosis may be considered as recurrent case (no clear physiological reference period) |
Use of data
Rapid feedback is essential for farmers and veterinarians to encourage the development of an efficient health monitoring system. Information can be provided soon after the data collection begins in the form individual farm statistics. If those results include metrics of data quality, then producers may have an incentive to quickly improve their data collection practices. Regional or national statistics should be provided as soon as possible as well. Early detection and prevention of health problems is an important step towards increasing economic efficiency and sustainable cattle breeding. Accordingly, health reports are a valuable tool to keep farmers and veterinarians motivated and ensure continuity of recording.
Direct and indirect observations need to be combined for adequate and detailed evaluations of health status. Reference should be made to key figures such as calving interval, pregnancy rate after first insemination, and non-return rate. A short time interval between calving and many diagnoses of fertility disorders is due to the high levels of physiological stress in the peripartum period, and also may indicate that a farmer is actively working to improve fertility in their herd. A low rate of reported mastitis diagnoses is not necessarily proof of good udder health, but may reflect poor monitoring and documentation.
In addition to recording disease events, on-farm system also can be used to record useful management information, such as body condition scores, locomotion scores, and milking speed (USDA, 2010[40]). Individual animal statuses (clear/possibly infected/infected) for infectious diseases such as paratuberculosis (Johne's disease) and leukosis also may be tracked. Such data may be useful for monitoring animal welfare on individual farms.
Improvement of management (individual farm level)
Farmers
Optimised herd management is important for economically successful farming. Timely availability of direct health information is valuable and supplements routine performance recording for early detection of problems in a herd. Therefore, health data statistics should be added to existing farm reports provided by milk recording organisations. Examples from Austria are found in Egger-Danner et al. (2007[41]) and Austrian Ministry of Health (2010).
Veterinarians
The EU-Animal Health Strategy (2007-2013), 'Prevention is better than cure', underscores the increased importance placed on preventive rather than curative measures. This implicates a change of the focus of the veterinary work from therapy towards herd health management.
With the consent of the farmer, the veterinarian can access all available information about herd health. The most important information should be provided to the farmer and veterinarian in the same way to facilitate discussion at eye-level. However, veterinarians may be interested in additional details requiring expert knowledge for appropriate interpretation. Health recording and evaluation programs should account for the need of users to view different levels of detail.
The overall health status of the herd will benefit from the frequent exchange of information between farmers and veterinarians and their close cooperation. Incorrect interpretation or poor documentation of health events by the farmer may be recognised by attending veterinarians, who can help correct those errors. Herd health reports will provide a valuable and powerful tool to jointly define goals and strategies for the future, and to measure the success of previous actions.
Immediate reactions
It is important that farmers and veterinarians have quick access to herd health data. Only then can acute health problems, which may be related to management, be detected and addressed promptly. An Internet-based tool may be very helpful for timely recording and access to data.
Long term adjustments
Less-detailed reports summarizing data over longer time periods (e.g., one year) may be compiled to provide an overview of the general health status of the herd. Such summary reports will facilitate monitoring of developments within farm over time, as well as comparisons among farms on district and/or province level. References for management decisions which account for the regional differences should be made available (Austrian Ministry of Health, 2010; Schwarzenbacher et al., 2010[42]). Definitions of benchmarks are valuable, and for improvement of the general health status it is important to place target oriented measures.
Monitoring of the health status (population level)
Ministries and other organisations involved in animal health issues are very interested in monitoring the health status of the cattle population. Consumers also are increasingly concerned about aspects of food safety and animal welfare. Regardless of which sources of health information are used, national monitoring programs may be developed to meet the demands of authorities, consumers and producers. The latter may particularly benefit from increased consumer confidence in safe and responsible food production.
It is recommended that all information, including both direct and indirect observations, be taken into account when monitoring activity and preparing reports. For example, information on clinical mastitis should be combined with somatic cell count or laboratory results.
It is extremely important to clearly define the respective reference groups for all analyses. Otherwise, regional differences in data recording, influences of herd structure and variation in trait definition may lead to misinterpretation of results. To ensure the reliability of health statistics it may be necessary to define inclusion criteria, for example a minimum number of observations (health records) per herd over a set time period. Such lower limits must account for the overall set-up of the health monitoring program (e.g., size of participating farms, voluntary or obligatory participation in health recording).
Key measures that may be used for comparisons among populations are incidence and prevalence. In any publication it must be clear which of the two rates is reported, and also how the rates have been calculated.
Incidence
Number of new cases of the disease or health incident in a given population occurring in a specified time period which may be fixed and identical for all individuals of the population (e.g., one year or one month) or relate to the individual age or production period (e.g., lactation = day 1 to day 305 in milk).
For example, the lactation incidence rate (LIR) of clinical mastitis (CM) can be calculated as the number of new CM cases observed between day 1 and day 305 in milk.
Equation 1. For computation of lactation incidence rate for clinical mastitis.
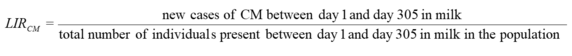
Another, and arguably a more accurate incidence rate could be calculated, by taking into account the total number of days at risk in the denominator population. This allows for the fact that some animals will leave the herd prematurely (or may join the herd late) and will therefore not contribute a 'full unit' of time of risk to the calculation.
Equation 2. For computation of lactation incidence rate for clinical mastitis taking account of day as risk.
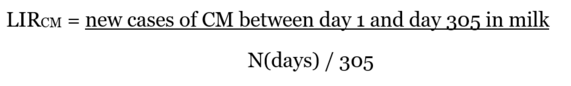
Where N(days) is the total number of days that individual cows were present in the herd when between 1 and 305 days in milk; ie a cow present throughout lactation will add 305 days, a cow culled on day 30 of lactation will only contribute 30 days etc., … (divided by 305 as that is the period of analysis).
Prevalence
Number of individuals affected by the disease or health incident in a given population at a particular point in time or in a specified time period.
Equation 3. For computation of prevalence of clinical mastitis.

Genetic evaluation (population level)
Traits for which breeding values are predicted differ between countries and dairy breeds. However, total merit indices have generally shifted towards functional traits over the last several years (Ducrocq, 2010[43]). Currently, most countries use indirect health data like somatic cell counts or non-return rates for genetic evaluation to improve health and fertility in the dairy population. Direct health information may be used in the future, and already has been included in genetic evaluations for several years in the Scandinavian countries (Heringstad et al., 2007[44]; Østeras et al., 2007[45]; Johansson et al., 2006[46]; Johansson et al., 2008[47]; Interbull, 2010[48]; Negussie et al., 2010[49]).
Trait definitions for genetic analyses must account for frequencies of health incidents, with low incidence rates requiring more records for reliable estimation of genetic parameters and prediction of breeding values. Broader and less-specific definitions of health traits may mitigate this problem, with a possible loss of selection intensity. However, obligatory plausibility checks of data must be performed as specifically as possible, and any combination of traits at a later stage must account for the pathophysiology underlying the respective health traits. Examples of trait definitions found in the literature are given together with the reported frequencies in Table 8.
Many studies have shown that breeding measures based on direct health information can be successful (e.g., Amand, 2006[50], Zwald et al., 2006a[51],b[52]; Heringstad et al., 2007[53]). When using indirect health data alone or in combination with direct health data it must be remembered that the information provided by the two types of traits is not identical. For example, the genetic correlations among clinical mastitis and somatic cell count are in the range of 0.6 to 0.7 depending on the definition of the indirect measure of mastitis (e.g., Koeck et al., 2010b[54]). Correlation estimates are lower for fertility traits, with moderately negative genetic correlation of -0.4 between early reproduction disorders and 56-day non-return-rate (Koeck et al., 2010a[55]).
Heritability estimates of direct health traits range from 0.01 to 0.20 and are higher when only first rather than all lactation records are used (Zwald et al., 2004[56]). Results from Fleckvieh and Norwegian Red indicate that heritabilities of metabolic diseases may be higher than heritabilities of udder, locomotory, and reproductive diseases (Zwald et al., 2004[57]; Heringstad et al., 2005[58]). When comparing genetic parameter estimates, methodological differences such as the use of linear versus threshold models need to be considered.
Existing genetic variation among sires with respect to functional traits can be used to select for improved health and longevity. Experience from the Scandinavian countries shows that genetic evaluation for direct health traits can be successfully implemented. For several disease complexes it may be advantageous to combine direct and indirect health data (e.g. Johansson et al., 2006[59], Johanssen et al., 2008[60], Negussie et al., 2010[61], Pritchard et al., 2011 [62]and Urioste et al., 2011[63]; Koeck et al., 2012a[64],b[65]).
Further information on already-established genetic evaluations for functional traits including considered direct and indirect health information can be found on the Interbull website (http://www.interbull.org/ib/geforms).
Examples of national genetic evaluations (2010)
| Table 8. Lactation incidence rates (LIR), i.e. proportions of cows with at least one diagnosis of the respective disease within the specified time period. | |||
| Breed trait | Time period (parities considered) |
LIR (%) | Reference |
| Danish Red | |||
| Udder diseases | -10 to 100 days in milk (1st lactation) |
22 | Nielsen et al., 2000[66] |
| Reproductive disturbances | 12 | ||
| Digestive and metabolic diseases | 3 | ||
| Feet and legs disorders | 6 | ||
| Danish Holstein | |||
| Udder diseases | -10 to 100 days in milk (1st lactation) |
21 | Nielsen et al., 2000[67] |
| Reproductive disturbances | 10 | ||
| Digestive and metabolic diseases | 3 | ||
| Feet and legs disorders | 6 | ||
| Danish Jersey | |||
| Udder diseases | -10 to 100 days in milk (1st lactation) |
24 | Nielsen et al., 2000[68] |
| Reproductive disturbances | 3 | ||
| Digestive and metabolic diseases | 2 | ||
| Feet and legs disorders | 4 | ||
| Norwegian Red | |||
| Clinical mastitis | -15 to 120 days in milk (1st, 2nd, 3rd lactation) |
15.8 19.8 24.2 |
Heringstad et al., 2005[69] |
| Milk fever | -15 to 30 days in milk (1st, 2nd, 3rd lactation) |
0.1 1.9 7.9 | |
| Ketosis | -15 to 120 days in milk (1st, 2nd, 3rd lactation) |
7.5 13.0 17.2 | |
| Retained placenta | 0 to 5 days in milk (1st, 2nd, 3rd lactation) |
2.6 3.4 4.3 | |
| Swedish Holstein | |||
| Clinical mastitis | -10 to 150 days in milk (1st, 2nd, 3rd lactation) |
10.4 12.1 14.9 |
Carlén et al., 2004 |
| Finnish Ayrshire | |||
| Clinical mastitis | -7 to 150 days in milk (1st, 2nd, 3rd lactation) |
9.0 10.6 13.5 |
Negussie et al., 2006[70] |
| Fleckvieh (Simmental) | |||
| Clinical mastitis | -10 to 150 days in milk | 9.6 | Koeck et al., 2010a[71] |
| Early reproductive disorders | 0 to 30 days in milk | 7.2 | Koeck et al., 2010a[72] |
| Late reproductive disorders | 31 to 150 days in milk | 8.2 | Koeck et al., 2010a[73] |
| Brown Swiss | |||
| Clinical mastitis | -10 to 150 days in milk | 8.4 | Koeck et al., 2010b[74] |
Disease Codes
A full list of disease codes is available:
- On the ICAR website here - https://www.icar.org/index.php/publications-technical-materials/amendments-recording-guidelines/diseases-codes-for-cows/ and,
- Can be downloaded as an .xlsx file here - https://www.icar.org/wp-content/uploads/2020/01/ICAR-Claw-Health-Key-coding-20180921.xls
Acknowledgments
This document is the result the ICAR working group on functional traits. The members of this working group at the time of the compilation of this Section were:
- Lucy Andrews, Holstein UK, Scotsbridge House Rickmansworth, Herts, WD3 3BB United Kingdom; lucyandrews@holstein-uk.org
- Andrew John Bradley, Quality Milk Management Services, United Kingdom; andrew.bradley@qmms.co.uk
- John B. Cole, Animal Improvement Programs Laboratory, USA; John.Cole@ARS.USDA.GOV
- Christa Egger-Danner, ZuchtData EDV-Dienstleistungen GmbH, Austria; egger-danner@zuchtdata.at (Chairperson since 2011)
- Nicholas Gengler, Gembloux Agricultural University, Belgium; gengler.n@fsagx.ac.be
- Bjorg Heringstad, Department of Animal and Aquacultural Sciences / Geno , Norwegian University of Life Sciences, Norway; bjorhe@umb.no
- Jennie Pryce, Victorian Departement of Primary Industries, Australia; jennie.pryce@dpi.vic.gov.au
- Katharina Stock, VIT, Germany; Friederike.Katharina.Stock@vit.de
- Erling Strandberg, Sweden (member and chairperson till 2011); Erling.Strandberg@slu.se
Frank Armitage, United Kingdom; Georgios Banos, Faculty of Veterinary Medicine, Greece; Ulf Emanuelson, Swedish University of Agricultural Science, Sweden; Ole Klejs Hansen, Knowledge Centre for Agriculture, Denmark and Filippo Miglior, Canadian Dairy Network, Canada and is thanked for their support and contribution. Rudolf Staufenbiel, FU Berlin, and co-workers is thanked for their contributions to standardization of health data recording.
Female Fertility in Dairy Cattle
Technical abstract
These guidelines are intended to provide people involved in keeping and breeding of dairy cattle with recommendations for recording, management and evaluation of female fertility. Aspects of bull fertility are covered by another set of ICAR guidelines (Section 6), compiled by the ICAR working group for Artificial Insemination. The guidelines described here support establishing good practices for recording, data validation, genetic evaluation and management aspects of female fertility.
To establish a recording scheme for female fertility the following data are desirable:
- Calving dates.
- All artificial insemination dates including natural mating dates where possible.
- Information on fertility disorders.
- Pregnancy test results.
- Culling data.
- Body condition score.
- Hormone assays.
Other novel predictors of fertility, such as activity based information (pedometer), are also growing in popularity.
This document includes a list of parameters for female fertility and information on recording and validating these data.
Introduction
In broad terms, "fertility" is defined as the ability to produce offspring. In the dairy industry, female fertility refers to the ability of a cow to conceive and maintain pregnancy within a specific time period; where the preferred time period is determined by the particular production system in use. The relevance of certain fertility parameters may therefore differ between production systems, and evaluations of female fertility data have to account for these differences.
There are currently significant challenges to achieving pregnancy in high yielding dairy cows. Accordingly, female fertility has received substantial attention from scientists, veterinarians, farm advisors and farmers. Culling rates due to infertility are much higher than two or three decades ago, and conception rates and calving intervals have also deteriorated. There is no doubt that selection for high yields, while placing insufficient or no emphasis on fertility, has played a role in declining rates of female fertility worldwide, because genetic correlations between production and fertility are unfavourable (e.g. Pryce & Veerkamp 1999[75]; Sun et al., 2010[76]). Most breeding programs have attempted to reverse this situation by estimating breeding values for fertility and including them with appropriate weightings in a multi-trait selection index for the overall breeding objective of dairy cattle.
One of the most important ways that fertility can be improved, through both management strategies and getting better breeding values is by collecting high quality fertility phenotypes. Female fertility is a complex trait with a low heritability, because it is a combination of several traits which may be heterogeneous in their genetic background. For example, it is desirable to have a cow that returns to cyclicity soon after calving, shows strong signs of oestrus, has a high probability of becoming pregnant when inseminated, has no fertility disorders and the ability to keep the embryo/foetus for the entire gestation period. For heifers, the same characteristics except the first one apply. Multiple physiological functions are involved including hormone systems, defense mechanisms and metabolism, so a larger number of parameters may reflect fertility function or dysfunction. However, in initiating a data recording scheme for female fertility it is often not practical (although desirable) to encompass all aspects of good fertility.
The obstacles that exist in adequate recording of fertility measures include: data capture i.e. handwritten notebooks versus computerized data recording and how these data link to a central database used to store data from multiple herds. Although many countries already have adequate fertility recording systems in place, the quality of data captured may still vary by herd. Many farmers are already motivated to improve fertility (as there is global awareness of the decline in dairy cow fertility over recent years). However, what is not always clearly understood is the importance of different sources of fertility data in providing tools that can be used to improve fertility performance.
The principles and type of data that should be recorded are the same regardless of the production system. However, the way in which the data are used i.e. the measures of fertility may vary according to the type of production system. For this reason, we have made a distinction between seasonal and non-seasonal herds:
In seasonal systems cows calve (typically) in the spring, so that peak milk production matches peak grass growth. An alternative is autumn calving herds that use feed conserved from pasture grown in the summer months. True seasonal systems have all cows calving as a tight time frame, i.e. within 8 weeks of the planned start of calvings.
In year-round-systems heifers calve for the first time (predominantly) at a certain age e.g. close to two years of age regardless of the month of year and calvings occur all through the year, so that the calving pattern appears to be reasonably flat.
Types and sources of data
Types of data
Calving dates
Calving dates can be used to calculate the interval between consecutive calvings and to confirm previously predicted pregnancies / conceptions.
To consider: In order to handle bias from culling it is useful to also record culling of cows and the culling reasons.
Insemination data
Data on inseminations can be used either alone or in combination with other data e.g. calving dates to define interval traits. Where the measure is initiated by a calving date, it can only be calculated for cows.
Insemination (and calving) dates can be used to calculate the following traits, those that can be measured for cows and/or heifers are indicated in brackets:
- Interval from calving to first insemination (cows).
- Interval from planned start of mating to first insemination (cows and heifers).
- Non-return rate (to first insemination or within a defined time period) (cows and heifers).
- Conception rate (to any insemination).
- Calving rate within a time period (an individual's phenotype is 0/1) (cows and heifers).
- Number of inseminations per lactation or insemination period (cows and heifers).
- Number of inseminations per calving or pregnancy.
- Interval from first to last insemination (cows and heifers).
- Interval between inseminations (cows and heifers).
- Interval from calving to last insemination (cows).
There is no best set of traits for evaluation of female fertility, but it is recommended to consider traits which reflect more than one aspect of fertility, e.g. interval from calving to first insemination or interval from calving to first oestrus (return to cyclicity) and non-return rate (probability of conception). For seasonal calving systems, submission rate and calving rate could be alternatives, refer to Table 9. However, calving interval (the interval between two calvings) requires the least data, only calving dates, and is often used as a first step to genetic evaluations for fertility in the absence of insemination or other fertility data. It has to be used with care as highlighted above.
Fertility disorders
These data are either diagnoses related to treatments by veterinarians or observations from farmers. Details can be found above in 1.9.1 above.
Milk production and composition data
Milk yield is correlated to fertility, and could be used as a predictor (for example in a multi-trait analysis of fertility). However, care should be taken, as the heritability of milk yield is high compared to fertility, the contribution of milk yield to the fertility breeding value could be considerable, making it difficult to identify bulls that are superior for both fertility and milk production. Results from selection based on Total Merit Indices show that it is possible to stabilize fertility if a certain weight is put on fertility.
Recent research confirmed genetic links between fertility and milk composition. In particular, changes of milk fatty acid profiles were identified (Bastin et al., 2011[77]) as useful predictors.
Results of pregnancy tests and further hormone assays
Pregnancy status can be determined by veterinary diagnosis, such as uterine palpation or ultrasound or by using information from hormones or circulating peptides associated with pregnancy. The timing of this data is important and should generally be done in consultation with veterinary practitioners. Other hormones, such as progesterone can be used to to determine the post-partum onset of cyclic activity and calculate e.g. interval from calving to first luteal activity (CLA) or other similar traits. The advantage of this trait is that compared with the interval from calving to first insemination, it is not influenced by the farmer's decision of when to start inseminations. However, it may be costly.
Heat strength
Physical activity increases during oestrus, in addition there are other behavioural changes, such as standing heat and mounting behaviour. These signs are used to detect oestrus and can be used to calculate traits such as interval between calving and resumption of oestrus. Tail paint (on the tail head) or colour ampoules attached to the tail head are used in some countries to aid oestrus detection. For larger herds, tail painting is used as a tool to aid insemination rather than resumption of cyclicity, however, on many farms, the decision to inseminate is often made after a defined period between calving and first insemination. In many practical situations it may be unrealistic to expect oestrus (without insemination) data to be collected, however recently there has been innovation in automating heat detection. For example, pedometers and more sophisticated activity monitors are now being used routinely on many farms as part of a management package. As cows become more active when in oestrus, the pedometer information needs to be compared to a baseline for the same cow and algorithms have been developed to interpret the data collected. The efficiency of oestrus detection rate has been reported to range between 50 and 100% depending on the criteria of success (At-Taras & Spahr, 2001). The gold-standard of oestrus detection are still progesterone measurements and imperfect concordance between pedometer and progesterone determined oestrus has been determined because activity monitors will not detect silent behavioural oestrus (Lovendahl & Chagunda, 2010). However, clearly there is an advantage in both progesterone and activity determined oestrus as they do not require farm observations.
Culling data
Culling data and culling reasons are important information especially if traits referring to longer time intervals (i.e. particularly those referring to calving dates) are used. Information on cows or heifers culled because of fertility disorders are of use, especially to remove bias arising from cows disappearing from the recording system i.e. a bull can have a biased proof if a lot of his daughters are culled for infertility and this is not recorded.
In the absence of accurate culling data, a useful proxy for monitoring fertility at the herd level is the proportion of animals failing to conceive by 300 days post calving. Cows not served by 300 days most likely reflect non-fertility culls, whereas cows that have been served and fail to conceive are more likely to reflect culls as a result of failure to conceive given that the majority of involuntary culls and decisions on planned culling occur in early lactation prior to the start of the breeding season.
Metabolic stress and body condition
Metabolic stress is defined as the degree of metabolic load that distorts normal physiological function. A distortion of normal physiological function may be temporary infertility, where the metabolic load is too great for the cow to invest in reproduction (future pregnancy) when the current lactation is not sustainable. Metabolic load is reflected by the stability of energy balance, which Veerkamp et al. (2001) [78]suggested was related to traits such as milk yield, body condition score (BCS) and live weight (LWT).
By itself live weight is not a particularly good measure of energy balance, as tall thin cows may have weights similar to smaller cows in better condition. Therefore, BCS has been favoured as an indicator for energy balance. Cows with low BCS may have health problems, such as metritis, which may be the underlying problem for poor fertility. However, most studies worldwide have shown that BCS is a good indicator of female fertility, as cows that are mobilize body tissue may be more likely to use this energy to sustain lactation instead of invest in a pregnancy. Therefore, BCS has been found to be suitable to be incorporated into selection indexes for fertility, such as in New Zealand (Harris et al., 2007[79]). BCS is sometimes measured as part of the linear type assessment in pedigree and progeny testing herds it can also be measured by the farmer. However, in some situations, use of BCS as a predictor trait for fertility has been found to be limited (Gredler et al., 2008[80]).
Sources of data
Female fertility data originates from different data sources which differ considerably with respect to information content and specificity; for example from veterinary practices, laboratories, milk recording organisations, breed associations and farms etc. Therefore, ideally, the data source should be clearly indicated whenever information on fertility status is collected and analysed. When data from different sources are combined, the origin of data must be taken into account. Regardless of the data source, it is desirable to have as few steps as possible from initial data recording.
Milk-recording
Initiation of lactation requires a calving date to be recorded for a cow. Calving dates are generally collected by organisations that are responsible for recording milk production, based on dates reported by the farmer, or more commonly gathered during the registration of births in countries operating mandatory birth registration systems. Calving dates are the most basic source of data available for evaluation of female fertility and can be used to determine calving intervals (defined as the number of days between two consecutive calvings).
Content
- Calving dates.
- Culling reasons.
Advantages
- Covers both cyclicity and conception.
- No additional effort for recording and therefore can be used as an easy first-step into evaluating fertility.
- Possible use of already-established data flow (reporting of calving).
Disadvantages
- Missing dates for cows with problems around calving that do not enter the herd for milk recording.
- Only available for cows, not for heifers.
- Calving interval data may be censored, as cows that are infertile are often culled before calving again. If specific culling reasons are available, then information on animals that are culled for infertility can be a very useful addition to calving interval data, as the least fertile cows (i.e. cows culled for infertility) can be distinguished from cows culled for other reasons.
AI organisations or producers
AI organisations and other AI operators record insemination dates and the AI sire used for the insemination. Inseminations can either be recorded in a logbook and later transferred to a computer or directly into a computer (sometimes handheld device).
Content
- Information on inseminations (date of insemination, sire/origin of semen, semen batch, inseminator e.g. technician or member of farm staff).
- Sexed semen, embryo transfer, straw splitting etc. should be noted.
- Interventions such as synchrony should also be recorded, as it is possible that this may affect analysis results.
Advantages
- If logistics for collection of insemination data are established, data can be collected from many farms.
- A broad range of measures of fertility can be calculated from insemination dates (often with calving dates) see Table 1. These measures can cover conception and cyclicity.
Disadvantages
- If logistics for collection of insemination data are not established, considerable efforts may be needed to set-up recording.
- Completeness of recording may vary, especially if there are no legal documentation requirements.
- In situations where farmers often use AI for a set period of time followed by natural mating to farm bulls, some mating dates will be missing.
Veterinarians
Veterinarians are often involved in monitoring herd fertility. Pregnancy diagnosis or pregnancy testing is practiced and recorded by many veterinary practices to confirm a pregnancy. Uterine palpation per rectum or ultrasonography at around day 60 of conception is a valuable source of data because it is more accurate than non-return rates. Treatment for fertility disorders should also be recorded. From the economic point of view, a cow with good fertility without any treatments needed may be clearly preferred over a cow that was treated several times before it got pregnant.
Content
- Pregnancy status.
- Diagnoses of fertility disorders.
Advantages
- Direct information on fertility, which is not covered by calving and insemination data.
Disadvantages
- Veterinary support and training needed to ensure data quality and consistency in diagnosis and definitions.
- Completeness of recording may vary depending on work peaks on the farm.
- Accurate animal identification may be an issue, as the data may be used (by the veterinary practice) to assess herd-level fertility rather than individual cow fertility.
- Data on pregnancy diagnosis may only be available for a subset of the herd.
On-farm computer software
Multiple herd management software packages are available for dairy farmers to record their own data. Some of this software interacts with the milk-recording organisations via standard interfaces, i.e. there are automatic exchanges of data between the central database and the computer on the farm. Farmers can enter calving, insemination, culling and pregnancy test information themselves. For genetic evaluation purposes, it is important that all the data is entered. Information on natural matings (if applicable) should also be recorded where possible and practical, which may not be the case for very large herds.
Content
- Insemination data.
- Calving data.
- Pregnancy test results.
Advantages
- No additional effort for recording.
- Continuous recording.
Disadvantages
- Very often only software solutions within farm, difficulties of standardized export of data, although many software packages ensure data exchange with the genetic evaluation unit is possible.
- Trait definitions may differ between systems, requiring source-specific data handling.
- Incompleteness of insemination data, for example in some cases only the last successful insemination may be recorded for management purposes
Data security
Data security is a universally important issue when collecting and using field data.
The legal framework for use of fertility data has to be considered according to national requirements and data privacy standards. The owner of the farm on which the data are recorded is the owner of the data, and must enter into formal agreements before data are collected, transferred, or analysed.
Documentation
Documentation is the precondition of use of fertility data for management and breeding purposes.
Pre-requisite information:
- Unique animal identification of both the cow and service sire.
- Unique herd identification.
- Ancestry or pedigree information (at the very least the cow's sire should be recorded).
- Birth registration.
- A central database (Often data is recorded on the farm's computer(s) and then uploaded to the milk recording agency who then transfer the data to a central database. Alternatively, data can exchange directly between the farm computer and the central database).
Useful additional documentation:
- Individual identification of the recording person.
- Details on respective fertility event.
- Artificial insemination or natural service.
- Type of semen used (e.g. sexed semen, fresh semen).
- Type of recording and method of data transfer (software used for on-farm recording, online-transmission).
The systematic use and appropriate interpretation of fertility data requires that different types of information can be combined such as date of birth, sex, breed, sire and dam, farm/herd; calving dates, and performance records. Therefore, unique identification of the individual animals used for the fertility database must be consistent with the animal ID used in existing databases (for more details see the "ICAR rules, standards and guidelines on methods of identification").
Data that can be used to calculate female fertility measures can originate from a number of sources including farm software, milk-recording organisations, veterinarians, breed societies and laboratories. Ideally, as much data as possible should be recorded electronically, as this reduces transcription errors. As long as data is as error free as possible, the origin of data is less important. However, it is preferable for data to be transferred to a central database in as few steps as possible and as quickly as possible. Genetic evaluation of young bulls relies on early information on fertility being available.
Recording of female fertility
Stepwise decision support for recording fertility
In setting up a recording scheme or using data for genetic evaluation of fertility, the data that is currently captured needs to be considered in addition to implementing strategies for including other data. For example, calving dates and consequently calving interval, is the most basic measure of fertility. Then, insemination dates can be added, to calculate interval traits and non-return rates. Ideally, pregnancy test results should also be recorded as these can be used as early indicators of conception. Finally, or in some cases alternatively, other predictors, such as fertility disorders, type traits, culling reasons and measures derived from hormones assays can also be added.
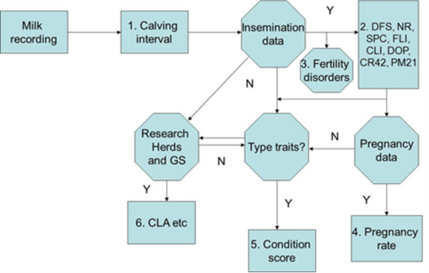
- If only data from a milk recording organisation is available, then calving interval can be measured as the interval between 2 successive calvings.
- If insemination data is available then days to first service (DFS), non-return (NR), number of services per conception (SPC), first to last service interval (FLI), calving to last insemination (CLI), days open (DOP) can be measured. Conception within 42 days of the planned start of mating and presented for mating within 21 days of the planned start of mating are measures suitable for seasonal systems and require a day when inseminations were started in the breeding season to be identified. Similarly first service submission can be used if a voluntary wait period is defined.
- If information about fertility disorders (diagnoses) are available, the information about cows with e.g. cystic ovaries, silent heat, metritis, retained placenta or puerperal diagnoses can be included in an fertility index.
- If pregnancy test/diagnosis data is available, then conception or pregnancy to the first (or second) insemination can be calculated, or in seasonal systems, conception within 42 days of the planned start of mating.
- If type data is recorded regularly across parities, body condition score (a measure of fatness and metabolic status) can be evaluated. The limitation with condition score as part of a type classification scheme is that it is generally only recorded once, often on only selected cows, and therefore its usefulness may be limited.
- If there are research herds or dedicated nucleus herds available, then commencement of luteal activity can be measured on a subset of animals (reference population). If these animals are also genotyped, then a genomic prediction equation can be calculated that can be applied to animals with genotypes but not phenotypes.
Data quality
General aspects
- Recorded data should always be accompanied by a full description of the recording program.
- If herds were selected how was this done?
- How were the people involved in recording (e.g., veterinarians, and farmers) selected and instructed? Any standardized recording protocol used?
- What types of recording forms or (computer) programs were used? - What type of equipment was used?
Is there any selection of animals within herds? Consistency, completeness and timeliness of the recording and representativeness of the data compared to the national population is of utmost importance. The amount of information and the data structure determine the accuracy of the data; measures of this accuracy should always be provided.
General quality checks
National evaluation centers are encouraged to devise simple methods to check for logical inconsistencies in the data. Examples of data checks include:
- The recording farm must be registered or have a valid herd-testing identification.
- The animal must be registered to the respective farm at the time of the fertility event.
- The date of the fertility event must refer to a living animal (must occur between the birth and culling dates), and may not be in the future.
- A particular insemination must be plausible. For example are the insemination dates impossible? (e.g. before the calving or birth date)
Continuity of data flow. Keys to long-term success
Regardless of the sources of fertility data included, long-term acceptance of the recording system and success of the fertility improvement program will rely on the sustained motivation of all parties involved. Quantifying the benefits of data recording of these data is important. For example, data can be useful information for herd management, but also genetic evaluation and integration of these traits into selection programs.
Trait definition
Refer to Table 9.
Calving interval
Calving interval is the number of days between two consecutive calvings. Calving interval covers both return to cyclicity and conception, however its main disadvantage is that it is sometimes biased because cows with the worst fertility are often culled early and hence do not re-calve. Calving interval is also available later than many other measures of fertility, so is not as useful for selection decisions.
Days Open
Days open is the interval between calving and the last insemination date. It is similar to calving interval provided the cow conceives to the last insemination, in which case days open is calving interval minus the gestation length. The USA currently calculates daughter pregnancy rate as 21/(Days Open - voluntary waiting period + 11). The voluntary waiting period is the period after calving that a farmer deliberately does not inseminate the cow.
Non-return rate
Non-return rate is a binary measure of whether a new mating or insemination event occurs after the first insemination within a time period. Frequently studied intervals are 28 days (NR28), 56 days (NR56) or 90 days (NR90). The reference period recommended by Interbull is 56 days. This trait can be evaluated for both heifers and cows.
Interval from calving to first insemination
The number of days between calving and first insemination is sometimes influenced by management aspects and this needs to be considered in fertility evaluations. However, it does provide a measure of return to cyclicity post-calving. However, it does not provide information on conception (Table 9).
Interval between 1st insemination and conception
The number of days between first insemination and positive pregnancy diagnosis.
Conception rate
Success or failure to conceive after each AI (this can be evaluated for heifers and cows)
Calving rate, e.g. 42 or 56 days, from planned start of calving (seasonal systems)
The binary measure of whether a cow returns 42 or 56 days from the herd's planned start of mating. It is generally confirmed by the presence of a subsequent calving date. A herd's planned start of mating is when artificial inseminations for the herd commence.
Number of inseminations per series
The number of inseminations in a lactation or within a certain time period (this can be evaluated for heifers and cows).
Heat strength
A subjective scale is often used for recording of heat strength. This scale could be divided in different ways and could have various numbers of classes, but the classes should be ordered in intensity. As an example, the Swedish system has a five-point scale (very weak, weak, clear signs, strong, very strong heat signs) where each point is described in more detail regarding physical signs of the vulva and mounting/being mounted.
Submission rate
The percentage of cows mated in a fixed number of days after the herd's start of mating. On an individual cow basis, recording is a binary score i.e. AI'd within a period of days from the herd's start of mating.
Fertility disorders - treatments for fertility disorders
Information on specific fertility disorders can provide valuable information for evaluation of female fertility. Recording details can be found in the ICAR Health guidelines.
Body condition score
The Body Condition Score (BCS) measures the fatness of the cow, especially in the region of the loin, hip, pinbone, and tailhead areas. Change in BCS in early lactation may be a better indicator of fertility compared with single observations of BCS per parity. To consider change in BCS it has to be recorded at least twice in early lactation and requires the dates of measurement.
Overview over traits
For monitoring the health status of dairy cows, an assessment of fertility is also useful to ensure that a complete picture of the health of the herd is available. For more information see the ICAR Health Guidelines.
| Table 9. Various traits used or possible to use and their potential relation to various aspects of cow fertility. | |||||||
| Ref. | Trait description | Aspect | System | ||||
| Return to cyclicity | Oestrus signs | Prob. of conception | Ability to keep embryo | Seasonal | Yearly | ||
| 1 | Interval between two consecutive calvings (calving interval) | + | + | + | + | ? | ? |
| 2 | Days open, interval from calving to conception (or last insemination) | + | + | + | + | ? | |
| 3 | Non-return rate (56, 128, .. days) | ++ | + | ? | ? | ||
| 4 | Interval from calving to first insemination | ++ | + | ? | |||
| 5 | Interval from first ins. to conception (or last insemination) | + | ++ | + | ? | ||
| 6 | Conception to 1st insemination (determined with pregnancy diagnosis) | ++ | + | ? | |||
| 7 | Calving rate (e.g. 42 or 56 days) from planned start of calving | ++ | ++ | ? | |||
| 8 | Number of ins. per series | + | ++ | + | ? | ||
| 9 | Heat strength | + | ? | ? | |||
| 11 | Treatments for fertility problems | + | + | + | ? | ? | |
| 12 | Body condition score, live weight change during early lact., energy balance | + | + | + | + | ? | ? |
| Submission rate: e.g., interval from planned start of mating to first insemination | ++ | + | ? | ||||
| Interval from calving to first luteal activity | ++ | ? | ? | ||||
| Interval between inseminations | + | (+) | ? | ||||
The number of + indicates how well the measure relates to the aspect of fertility
? indicates the suitability of the measure to the production system
Use of data
Improvement of management (individual farm level)
Although these guidelines focus mainly on evaluation of female fertility for genetic improvement, information is also very useful for on-farm decision-support. Routinely recording of fertility data allows the presentation of key figures for veterinary herd management.
Farmers
Optimised herd management is important for financially successful farming
Results of recording can be presented per individual animal or about cohorts and distinguish between retrospective "outputs" such as calving index and "inputs" such as number of services, results of pregnancy diagnosis in order to analyze overall performance (Breen et al., 2009[81]).
However, for short term decisions (e.g. whether to continue to inseminate or not) on-farm recording of fertility is probably the only practical solution. More sophisticated decision support may include correction of the observed level for systematic environmental effects (such as parity or stage in lactation) and time analysis. Fertility reports summarizing the fertility performance of age-groups within the dairy herd also allows farmers to benchmark their farm to others.
Timely availability of fertility information is valuable and supplements routine performance recording for optimised fertility management of the herd. Therefore, fertility data statistics should be added to existing farm reports provided by milk recording organisations. Examples from Austria are found in the Austrian Ministry of Health (2010).[82]
Immediate reactions
It is important that farmers and veterinarians have quick and easy access to herd fertility data. Only then can acute fertility problems, which may be related to management, be detected and addressed promptly. An Internet-based tool may be very helpful for timely recording and access to data. Lists of actions with animals ready to be inseminated or pregnancy tested are helpful.
Long term adjustments
Less-detailed reports summarizing data over longer time periods (e.g., one year) may be compiled to provide an overview of the general fertility status of the herd. Such summary reports will facilitate monitoring of developments within farm over time, as well as comparisons among farms on district and/or province level (Breen et al., 2009[83]; Austrian Ministry of Health, 2010[84]). Publication of key figures on female fertility at herd level will provide decision support at the tactical level. A general recommendation is to present recent averages (last year), but also to present trend over several years. If available, it is advised to include a comparison of the averages with a mean of a larger group of (similar) farms. For example, the average days open might be compared with the average days open for all farms in the same region or with the same milk production level.
Farm averages might also be specified for different groups of animals at the farm. For example, days open might be presented as an average for first lactation cows versus later parity animals. This denotes which groups require specific attention in the preventive management.
Definitions of benchmarks are valuable, and for improvement of the general fertility status it is important to place target oriented measures.
Monitoring of the health status (population level)
Government bodies and other organisations involved in animal health issues are very interested in monitoring the health status of the cattle population. Consumers also are increasingly concerned about aspects of food safety and animal welfare. Regardless of which sources of health information are used, national monitoring programs may be developed to meet the demands of authorities, consumers and producers. The latter may particularly benefit from increased consumer confidence in safe and responsible food production.
Fertility data is also important for providing genetic evaluations, both within country and between countries. The following section is from the Interbull website (http://www.interbull.org/ib/idea_trait_codes) and are the traits that the Interbull Steering committee chose in August 2007 to become part of MACE evaluations of fertility. Interbull considers female fertility traits classified as follows:
- T1 (HC): Maiden (H)eifer's ability to (C)onceive. A measure of confirmed conception, such as conception rate (CR), will be considered for this trait group. In the absence of confirmed conception an alternative measure, such as interval first-last insemination (FL), interval first insemination-conception (FC), number of inseminations (NI), or non-return rate (NR, preferably NR56) can be submitted.
- T2 (CR): Lactating (C)ow's ability to (R)ecycle after calving. The interval calving-first insemination (CF) is an example for this ability. In the absence of such a trait, a measure of the interval calving-conception, such as days open (DO) or calving interval (CI) can be submitted.
- T3 (C1): Lactating (C)ow's ability to conceive (1), expressed as a rate trait. Traits like conception rate (CR) and non-return rate (NR, preferably NR56) will be considered for this trait group.
- T4 (C2): Lactating (C)ow's ability to conceive (2), expressed as an interval trait. The interval first insemination-conception (FC) or interval first-last insemination (FL) will be considered for this trait group. As an alternative, number of inseminations (NI) can be submitted. In the absence of any of these traits, a measure of interval calving-conception such as days open (DO), or calving interval (CI) can be submitted. All countries are expected to submit data for this trait group, and as a last resort the trait submitted under T3 can be submitted for T4 as well.
- T5 (IT): Lactating cow's measurements of (I)nterval (T)raits calving-conception, such as days open (DO) and calving interval (CI).
Based on the above trait definitions the following traits have been submitted for international genetic evaluation of female fertility traits.
Acknowledgments
This document is the result of the work of the ICAR Functional Traits Working Group. The members of this working group are, in alphabetical order:
- Lucy Andrews, Holstein UK, Scotsbridge House Rickmansworth, Herts, WD3 3BB United Kingdom.
- Andrew John Bradley, Quality Milk Management Services, United Kingdom.
- John B. Cole, Animal Improvement Programs Laboratory, USA.
- Christa Egger-Danner, ZuchtData EDV-Dienstleistungen GmbH, Austria; (Chairperson of the ICAR Functional Traits Working Group since 2011)
- Nicolas Gengler, Gembloux Agro-Bio Tech, University of Liège, Belgium.
- Bjorg Heringstad, Department of Animal and Aquacultural Sciences / Geno , Norwegian University of Life Sciences, Norway.
- Jennie Pryce, Agriculture Victoria Research, Victoria, Australia
- Katharina Stock, VIT, Germany.
- Erling Strandberg, Swedish University of Agricultural Science, Uppsala, Sweden.
The working group acknowledges the valuable contributions and support in improving this document of Brian Wickham (ICAR) and Pavel Bucek (Czech-Moravian Breeders' Corporation), Stephanie Minery (Idele, France), Pascal Salvetti (UNCEIA), Oscar Gonzalez-Recio and Mekonnen Haile-Mariam (DEPI, Melbourne, Australia) and John Morton (Jemora, Geelong, Australia).
Udder health in Dairy Cattle
General concepts
Reader instructions
These guidelines are written in a schematic way. Enumeration is bulleted and important information is shown in text boxes. Important words are printed bold in the text.
The aim of these guidelines is to provide dairy cattle breeders involved in breeding programmes with a stepwise decision-support procedure establishing good practices in recording and evaluation of udder health (and correlated traits). These guidelines are prepared such that they can be useful both when a first start to the breeding programme is to be made, or when an existing breeding programme is to be updated. In addition, these guidelines supply basic information for breeders not familiar (inexperienced or ‘lay-persons’) with (biological and genetic) backgrounds of udder health and correlated traits.
Aim of these guidelines
Stepwise decision-support in developing a recording and evaluation system for udder health,
to support a genetic improvement scheme in dairy cattle.
Structure of these guidelines
These guidelines are divided in four parts:
- General introduction including a summary of the main principles.
- Background information on udder health and correlated traits.
- Stepwise decision-support for recording udder health and correlated traits.
- Stepwise decision-support for genetic evaluation of udder health and correlated traits.
The experienced animal breeder using these guidelines should read chapter 1 and is advised to read the text boxes of section 3.4 below. The inexperienced user is advised to read the full text of section 3.4 below.
General introduction
A healthy udder can be best defined as an udder that is ‘free from mastitis’. Mastitis is an inflammatory response, generally presumed to be caused by a bacterium.
| A healthy udder is an udder free from inflammatory responses to microorganisms. |
Mastitis is generally considered as the most costly disease in dairy cattle because of its high incidence and its physiological effects on e.g. milk production. In many countries breeding for a better production in dairy cattle has been practised for years already. This selection for highly productive dairy cows has been successful. However, together with a production increase, generally udder health has become worse. Production traits are unfavourably correlated with subclinical and clinical mastitis incidence.
A decreased udder health is an unfavourable phenomenon, because of several costs of mastitis like e.g. veterinary treatment, loss in milk production and untimely involuntary culling. Mastitis also implies impaired animal welfare.It is important to reduce the incidence of mastitis, because of production efficiency and animal welfare
| It is important to reduce the incidence of mastitis, because of production efficiency and animal welfare |
There is little hope that mastitis will be eradicated or an effective vaccine developed. The disease is much too complex. However, reducing the incidence of this disease is possible. An important component in reducing the incidence of mastitis is breeding for a better resistance. Dairy cattle breeding should properly balanced selection emphasis on production traits (milk and beef) and functional traits (such as fertility, workability, health, longevity, feed efficiency). This requires good practices for recording and evaluation of all traits - see table for an overview. These guidelines support establishing good practices for recording and evaluation of udder health. Decision-support for other trait groups will be subject of other guidelines developed by the ICAR working group on Functional Traits.
Operational situation breeding value prediction to be aimed for in dairy cattle genetic improvement schemes (source Proceedings International Workshop on Genetic Improvement of Functional Traits in cattle (GIFT) - breeding goals and selection schemes (7-9 November 1999, Wageningen, the Netherlands).
| Table 10. Breeding goal trait for which predicted breeding values should be available on potential selection candidates. | ||
|---|---|---|
| Trait group | Trait | |
| Milk production | Milk/carrier kg | |
| Fat kg or % | ||
| Protein kg or % | ||
| Milk quality | e.g., κ-casein | |
| Beef production | Daily gain/final weight | |
| Dressing or Retail % | ||
| Muscularity | ||
| Fatness, marbling | ||
| Calving ease | Direct effect | Parity split |
| Maternal effect | ||
| Still birth | ||
| Udder health | Udder conformation | a.o. Udder depth, teat placement |
| Somatic Cell Score | ||
| Clinical incidence | ||
| Female Fertility | Non-return rate | Age 1st calving, heat detectability, luteal activity |
| Interval Calving – 1st insemination | ||
| Male Fertility | ||
| Feet and legs problems | Conformation | Foot angle, Rear legs set |
| Locomotion | ||
| Clinical incidence | ||
| Workability | Milk speed, ability, leakage | |
| Temperament/Character | ||
| Longevity | Functional, residual | |
| Other diseases | Ketosis, metabolic problems | |
| Persistency | ||
| Metabolic stress/Feed efficiency | Mature weight Feed intake capacity Condition Score Energy Balance |
|
Recording
Selection on udder health starts with recording. Only by recording it is possible to differentiate in (predicted) breeding values for udder health between potential selection candidates. Mastitis can be recorded directly and indirectly.
Directly recorded mastitis is for example the number of clinical mastitis incidents per cow per lactation. The same can be done with subclinical mastitis, but this is mostly put on a par with recording of somatic cell count. Other traits for indirectly recording mastitis are milkability and udder conformation traits (e.g. udder depth, fore udder attachment, teat length).
| Table 11. Recording udder health. | |
| Direct | Indirect |
| Clinical mastitis incidents | Somatic cell count |
| Subclinical mastitis incidents | Milkability |
| Udder conformation traits | |
Clinical mastitis is an outer visual or perceptible sign of an inflammatory response of the udder: painful, red, swollen udder. The inflammatory response can also be recognised by abnormal milk, or a general illness of the cow, with fever. Sub-clinical mastitis is also an inflammatory response of the udder, but without outer visual or perceptible signs of the udder. An incident of sub-clinical mastitis is detectable with indicators like conductivity of the milk, NAG-ase, cytokines and somatic cell count in the milk.
Prerequisites
Recording and evaluation of udder health requires measuring direct and indirect traits, but also basic information is necessary. With an existing breeding programme to be updated with udder health, this prerequisite information is generally available, which might not be the case when starting with a new breeding programme.
Prerequisite information
- Unique animal identification and registration.
- Unique herd identification and registration.
- Individual animal pedigree information.
- Birth registration.
- A well functioning central database.
- Milk recording system (time information and logistics of sampling milk samples).
Evaluation
The recorded data from different farms should be combined to serve as a basis for a genetic evaluation of potential selection candidates in the genetic improvement scheme (per region, country or internationally). A genetic evaluation requires data to be recorded in a uniform manner. There should be ample data for reliable breeding value estimation. The quality of genetic improvement depends on the quality of these estimated breeding values.
On the basis of the estimated breeding values, selection candidates will be ranked. Estimated breeding values will be available per (recorded) trait, or as a combined ‘udder health index’. Such an udder health index will be a weighted summation of estimated breeding values for recorded (direct and indirect) traits. A ranking of selection candidates on an udder health index facilitates a selection on those animals that contribute mostly to improve udder health, i.e., reduced mastitis incidence. Together with indexes for other important trait groups, the udder health index can be combined towards a broader, general merit or performance index used for overall ranking of selection candidates.
Example sire evaluation in the Netherlands
The table below (Table 12) shows the top 10 of bulls marketed world-wide with the highest estimated breeding value (EBV) for udder health (May 2002). This is on the basis of the calculations of the national Dutch organisation for cattle breeding (NVO). The formula below shows the calculation of the breeding values for udder health:
Equation 4. Example of calculation of the breeding values for udder health.
EBVUH = -6.603 x EBVSCC - 0.193 x (EBVms - 100) + 0.173 x (EBVud - 100)+ 0.065 x (EBVfua - 100) – 0.108 x (EBVtl -100) +100
where EBVUH : EBV for udder health, EBVSCC : EBV for somatic cell count at 2log‑scale; EBVms : EBV for milking speed; EBVud : EBV for udder depth: EBV for fore udder attachment; EBVtl : EBV for teat length
The Durable Performance Sum (DPS) is the Dutch basis for the overall ranking of bulls. The components of the DPS are production, health and durability. The Total Score is the total score of the conformation of the bulls. The components for this trait are type, udder conformation and feet & legs.
| Table 12. Top ten bulls ranked for udder health (May 2002). | |||
| Name bull | Durable performance sum | Total score conformation |
Udder health index |
| Suntor magic | 52 | 107 | 115 |
| Carol prelude mtoto et | 217 | 112 | 111 |
| Wranada king arthur | 97 | 109 | 111 |
| Caernarvon thor judson-et | 87 | 107 | 111 |
| Mar-gar choice salem-et *tl | 65 | 108 | 111 |
| Prater | 51 | 112 | 111 |
| Ramos | 192 | 108 | 110 |
| Ds-kirbyville morgan-et | 165 | 108 | 110 |
| Whittail valley zest et | 158 | 104 | 110 |
| V centa | 129 | 112 | 110 |
Example sire evaluation in Sweden
Estimated breeding values for Swedish bulls for production, health and other functional Traits, sorted on mastitis (February 2002).
| Name bull | Total Merit Index | Production index | Milk (kg) | Production traits | ||
| Protein (kg) | Fat (kg) | Daily gain | ||||
| G Ross | 14 | 107 | 103 | 106 | 106 | 97 |
| Botans | 18 | 119 | 113 | 119 | 115 | 92 |
| Stöpafors | 12 | 108 | 105 | 108 | 106 | 98 |
| Inlag-ET | 13 | 106 | 106 | 106 | 109 | 96 |
| Torpane | 11 | 101 | 100 | 100 | 109 | 106 |
| Flaka | 21 | 111 | 112 | 111 | 114 | 111 |
| Bredåker | 14 | 106 | 100 | 105 | 113 | 104 |
| Brattbacka | 14 | 108 | 95 | 107 | 109 | 97 |
| Stensjö-ET | 20 | 118 | 115 | 117 | 123 | 105 |
| Name bull | Health traits | |||||
| Dau. fert. | Calvings | Mast. Resist. | Other diseases | Longevity | ||
| S | MGS | |||||
| 96 | 108 | 96 | 110 | 97 | 106 | |
| 97 | 104 | 97 | 108 | 100 | 104 | |
| G Ross | 95 | 89 | 98 | 106 | 100 | 111 |
| Botans | 105 | 106 | 108 | 104 | 103 | 106 |
| Stöpafors | 105 | 97 | 105 | 104 | 103 | 119 |
| Inlag-ET | 107 | 115 | 110 | 104 | 99 | 115 |
| Torpane | 108 | 96 | 107 | 103 | 103 | |
| Flaka | 104 | 106 | 102 | 103 | 108 | 112 |
| Bredåker | 100 | 106 | 103 | 102 | 98 | 107 |
| Name bull | Functional traits | ||||
| Stature | Legs | Udder | Milk speed | Tempr | |
| 102 | 111 | 105 | 89 | 105 | |
| 97 | 96 | 101 | 102 | 102 | |
| G Ross | 108 | 101 | 107 | 105 | 101 |
| Botans | 96 | 103 | 103 | 107 | 98 |
| Stöpafors | 103 | 97 | 105 | 108 | 96 |
| Inlag-ET | 100 | 101 | 97 | 92 | 98 |
| Torpane | 104 | 103 | 104 | 105 | 96 |
| Flaka | 97 | 99 | 104 | 92 | 96 |
| Bredåker | 94 | 94 | 100 | 110 | 107 |
Detailed information on udder health
Reader instruction
This chapter (3.9) gives background information on udder health and correlated traits. It is about direct (clinical mastitis) and indirect traits (somatic cell count, milkability and udder conformation traits). For the experienced reader reading only the bold printed words and text boxes should be sufficient.
Infection and defence
The first line of defence against an infection of microorganisms is the mechanical prevention of the mammary gland. This mechanical prevention is opposite to the ease of microorganisms to enter the teat canal: the easier the entrance, the weaker the mechanical prevention. The quality of this defence is related to the milkability and the udder conformation traits, like e.g. teat length and udder depth. However, when microorganisms enter the mammary gland, then the immune system causes an attraction of leukocytes to the place of infection, which results in an enlarged somatic cell count. So, a short-term increase in somatic cell count with or without accompanying clinical signs are on one hand a symptom of a failing first line of defence, but on the other hand indicating an appropriate immunological reaction. The picture below (Figure 2) shows the infection process, together with the destruction of a milk-secreting cell.
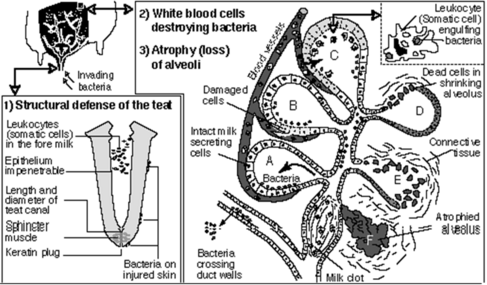
|
Mastitis causing bacteria
Contagious mastitis
It is caused by:
The S. aureus bacterium is hardly eradicable, but can be reduced to less than 5% of the cows in a herd. The S. agalactiae is fully eradicable from a herd.
It is caused by:
|
Clinical and subclinical mastitis
Mastitis can be subdivided in clinical and subclinical mastitis. Clinical mastitis is mastitis with outer visual or perceptible signs of the udder or the milk. Clinical mastitis is observed as abnormal milk, like flaky, clotted and / or “watery” milk. Possible perceptible signs on the udder are redness, painfulness and swollenness with fever.
Subclinical mastitis is not perceptible directly by a farmer or veterinarian, but is detectable with indicators. The most used indicator is the number of somatic cells per ml milk (somatic cell count). Other, less practised physiological indicators of subclinical mastitis are electrical conductivity of the milk, N-acetyl-ß-D-glucosaminidase, bovine serum albumin, antitrypsin, sodium, potassium and lactose content.
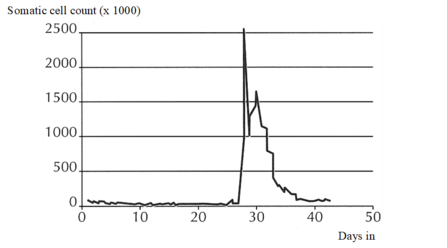
The somatic cell count is the most widely accepted criterion for indicating the udder health status of a dairy herd. An enlarged number of somatic cells in milk, which is unfavourable, points to a defence reaction.
Somatic cells in milk are primarily leukocytes or white blood cells along with sloughed epithelial or milk secreting cells. White blood cells are present in milk in response to tissue damage and/or clinical and subclinical mastitis infections. These cell numbers increase in milk as the cow’s immune system works to repair damaged tissues and combat mastitis-causing organisms. As the degree of damage or the severity of infections increase, so does the level of white blood cells. Epithelial cells are always present in milk at low levels. They are there as a result of a natural process inside the udder whereby new cells automatically replace old tissue cells. Epithelial cells result in normal milk SCC levels of <50,000.
The recommended industry standard for bulk SCC on delivery is one that is consistently <200,000. Many herds, which are successful in maintaining a herd SCC <100,000, have minimal to no mastitis infections.
| The somatic cell count is the number of somatic cells per millilitre of milk. Normal milk has less than 200,000 cells per millilitre. |
So, somatic cells are partly white blood cells or body defence cells whose primary functions are to eliminate infections and repair tissue damage. Somatic cell levels or numbers in the mammary gland do not reflect the whole pool of cells that can be recruited from the blood to fight infections. Somatic cells are sent in high numbers only when and where they are needed. Therefore, high SCC indicates mammary infection. A certain number of cells is necessary once an infection invades the udder. Together with a favourite low SCC, the speed of cell recruitment to the mammary gland and the cell competency are the major factors in infection prevention.
Aspects of recording clinical and sub-clinical mastitis
Recording clinical mastitis is possible but not common practice (yet). Scandinavian countries are the only countries that include mastitis incidence directly in their national recording and evaluation programs. However, other countries are working on a national recording and evaluation scheme for mastitis incidence as well. Reasons for increased interest in recording clinical mastitis are in
- Veterinary farm management support (i.e., identification of diseased animals and establishing treatment procedure).
- National veterinary policy-making (i.e., drugs regulations and preventive epidemiological measures).
- Citizens’ and consumers’ concerns about animal health and welfare and product quality and safety (i.e., chain management, product labelling).
- Genetic improvement (i.e., monitoring genetic level of the population and selection and mating strategies).
It is to be emphasised that recording of clinical mastitis is difficult, as it requires a clear definition (as given in these guidelines), an accurate administration with for example dates of incidence and (unique) cow numbers. It is also important that the reasons for recording are made clear to stakeholders and that information is not only gathered centrally, but also processed to obtain clear information for farm management support to be reported back to the farmer.
The (phenotypic) occurrence of clinical or subclinical mastitis is influenced by the genetic merit of the animal (its breeding value) and by environmental effects. When considering the total phenotypic variance between animals, for clinical mastitis about 2-5 % is because of genetic differences between the animals. The remaining differences between animals are because of different environmental influences and measuring errors. Known systematic environmental influences are for example in parity of the cow or stage in lactation. An evaluation of udder health traits will have to carefully consider these systematic environmental influences.
| On-farm management decision-support
Although these guidelines focus on evaluation of udder health for genetic improvement, information is also very useful for on-farm decision-support. Routinely recording of clinical incidents and somatic cell count allows the presentation of key figures for veterinary herd management. Operational - individual animal level Results of recording can be presented per individual animal. To support decision making, a note can accompany the presentation of the recording level when the level is above a certain threshold. For example, a SCC above 200,000 indicates that the cow may suffer from subclinical mastitis and requires treatment or it is advised to perform a bacteriological culturing. An additional listing might provide a direct overview of cows with attention levels for which further action is advised. More sophisticated decision support may include correction of the observed level for systematic environmental effects (such as parity or stage in lactation) and time analysis. Mastitis caused by different bacteria requires different preventive and curative measurements to be taken. Therefore, information from bacteriological culturing is generally very important in operational farm management. Tactical - herd level Publication of key figures on mastitis incidence, bacteriological culturing and SCC at herd level will provide decision support at the tactical term. A general recommendation is to present recent averages, but also to present the course of the averages over a longer time period. If available, it is advised to include a comparison of the averages with a mean of a larger group of (similar) farms. For example, the average on SCC might be compared with the average bulk somatic cell count for all farms delivering milk to the same factory. Farm averages might also be specified for different groups of animals at the farm. For example, SCC might be presented as an average for first lactation females versus later parity animals. This denotes which groups require specific attention in the preventive and curative management. |
Health card
In Norway, Finland and Denmark each individual cow has a health card, which is updated each time the veterinarian treats the animal. For example in Norway is a strict regulation of drugs such that all antibiotic treatments are carried out by the veterinary, and the farmer is not allowed treating his own animals. Completeness and consistency requires a very accurate administration; a condition in order to let a health card system be useful for breeding programs.
Quality control
In the Netherlands, it is now included in the ‘chain control on quality of milk’ that the farm is regularly visited by a veterinarian to record health status of the cows. This gives a ‘test-day’ comparison of all cows in the herd. This information can possibly be used for national veterinarian monitoring programmes and for selection programmes.
In many countries a reliable recording of clinical mastitis incidents is hard to achieve, which makes this trait not the first step in developing an udder health index. Somatic cell count (SCC) is genetically highly correlated with clinical mastitis: 0.60-0.70. This means, that when analysing field data, an observed high level of SCC is generally accompanied by a clinical mastitis event. In other words, although milk of healthy cows also shows variance in SCC, in day-to-day field data, most of the variance in SCC is caused by clinical mastitis events.
Given its high correlation to clinical mastitis, SCC is an appropriate indicator of udder health, as
- Somatic cell counts can be routinely recorded in most milk recording systems, giving better opportunities of accurate, complete and standardised observations.
- About 10-15% of the observed variation in scc is caused by differences in breeding values of the animals, which is higher than in clinical mastitis.
- It also reflects incidence of subclinical intramammary infections.
| Bulk somatic cell count
So far, we have considered SCC on animal level. In farm management also the average bulk somatic cell count (BSCC) is of interest. In many countries the BSCC is a basis for milk price payment by the dairy industry. The BSCC can also play a role in decision-support. High BSCC herds mainly deal with high levels of contagious, invasive organisms, which are mostly subclinical. Many cows are infected and substantial udder damage and milk losses are caused. When these infections become clinical, they are usually mild. Environmental infections are rarely seen because they are opportunists and can not compete with the highly invasive organisms. Low SCC herds have low levels of contagious, invasive pathogens. Thus, when they do have infections, they are usually environmental. Environmental infections are very vivid, with a severe illness and a possible death as a result. Environmental infections are not invasive, but opportunistic, thus most animals who get these are usually suppressed or heavily stressed, e.g. early lactation animals. A good management from the farmer can reduce the number of environmental infections. |
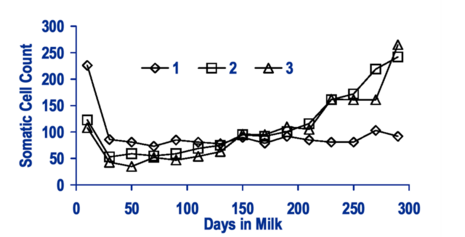
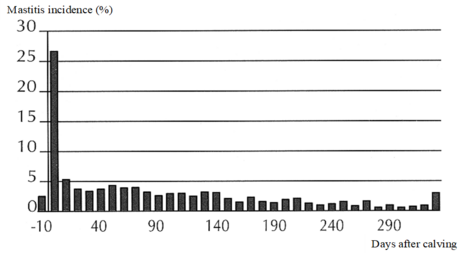
Relevance or lowering SCC
The importance of reducing clinical mastitis seems clear (high costs and impaired welfare), the importance of reducing subclinical mastitis might seem less obvious. However, there are several reasons for reducing the amount of subclinical mastitis (an increased number of somatic cells in milk (SCC)) in dairy cattle, like:
- Daughters of sires that transmit the lowest somatic cell score (log-transformation of somatic cell count) have lower incidence of clinical mastitis and fewer clinical episodes during first and second lactation.
- Decreased somatic cell count (SCC) has been shown to improve dairy product quality, shelf life and cheese yield. Increased SCC decreases cheese yield in two ways:
- By decreasing the amount of casein as a percentage of total protein in milk.
- By decreasing the efficiency of conversion of casein into cheese.
- High SCC in milk affects the price of milk in many payment systems that are based on milk quality.
- High SCC milk has a reduced flavour score because of an increase in salts.
Advantages of lowering somatic cell count
- Clinical mastitis: low incidence and few episodes.
- Improved dairy product quality.
- Higher milk prices.
Natural defence system
Part of the somatic cells is white blood cells - they are an essential part of the cow's immune system. Trying to lower the incidence of cases with highly increased somatic cell count (as an indicator that a defence reaction was necessary) is advised. Trying to lower somatic cell count below natural levels in milk of healthy cows is not advised. An essential part of the natural defence system is also the speed of white blood cells recruitment.
Milkability
There is an unfavourable genetic correlation between milkability (milking speed, milking ease or milk flow) and somatic cell count. Faster milking cows tend to have a higher lactation somatic cell count. In general, an unfavourable genetic correlation between milkability (i.e., milking speed) and udder health is assumed. This is explained by a possibly easier mechanical entry of pathogens into the udder associated with an easier exit of milk out of the udder ant teat canal.
However, some remarks are to be made with respect to this correlation between milkability and udder health.
Non-linearity
The genetic correlation is assumed to be non-linear. This means that at low and mediate levels of milking speed there is no influence on udder health. Only with extremely high milking speed, also observed as leakage of milk before milking time, the teat canal is too wide facilitating easy entrance of microorganisms.
Figure 7. A generalised representation of the milk low curve (Source: Dodenhoff et al., 2000).
Complete draining with milking.
With each milking, the last fraction of milk contains 3 to 10 times more cells than the first fraction. This however depends on the completeness of withdrawing milk from the udder, which itself is again related to milking speed. A higher milking speed, facilitates a more complete draining of the udder causing a higher SCC. This supports the suggestion that milking speed is unfavourably correlated with SCC but not with clinical mastitis.
Another important point is that milking speed is associated with the farmer’s labour time for milking. Increased milking speed per cow implies decreased costs for electrical power and decreased wear on milking equipment. Combining the two main aspects
- Reducing milking speed, or more specifically leakage as wanted because of udder health.
- Increasing milking speed because of reducing labour time
makes that milking speed is a trait with an intermediate, optimum level.
Recording of milking speed can be practised with advanced equipment. This advanced equipment can be:
- An additional equipment to be installed at regular intervals or at specific recording herds as part of a (national) recording programme for milking speed, or
- An integral part of the milking system at the farm, together with for example recording of milk conductivity, giving an integral, operational decision-support for the farmer in detecting cows with udder health problems.
An overall subjective scoring of milking speed can also be practised. The farmer can make a linear scoring of 1 very slow to 5 very fast (see also Section 05 of the ICAR Guidelines).
Udder conformation traits
Linear udder conformation is part of the recommended conformation recording in dairy cattle as approved by the World Holstein Friesian Federation (WHFF) and ICAR (see Section 05 of the ICAR Guidelines). Approved standard traits are:
Fore udder attachment Rear udder height
Median suspensory ligament Udder depth
Teat placement Teat length
A full description of these traits is given in 3.10.6 below. The reason for approval of this set of traits is based on the fact that each of these traits can have a predictive value for udder health, or the trait influences workability (and thus milking time). We therefore also recommend recording of udder conformation according to the ICAR/WHFF-recommendations.
Based on literature studies some indicative relative importance of the traits can be given. The udder conformation trait with the largest influence on udder health is the udder depth. Shallow udders appear to be obviously healthier than deep udders. A reason why shallow udders are healthier may be that deep udders have an increased exposure to pathogenic bacteria and are more likely to be injured.
Fore udder attachment also has an important influence on the udder health together with teat length. Probably again the main aspect here is that improved udder conformation (better attachment and shorter teats) decreases exposure to pathogens.
Again, also other traits are of importance, but the genetic relationship with udder health may be lower, and different traits may provide similar genetic information. This generally causes udder health indexes to be based on a limited number of udder conformation traits only.
Example age effect on udder conformation
| Table 13. The influence of age on udder conformation in Holstein Friesian and Jersey
(Source: Oldenbroek et al., 1993). | ||||
| Breed | Trait (cm) | Lactation number | ||
| 1 | 2 | 3 | ||
| Holstein | Distance rear udder-floor | 60.5 | 55.6 | 51.8 |
| Distance between front teat | 18.1 | 20.2 | 21.6 | |
| Jersey | Distance rear udder-floor | 51.2 | 47.5 | 44.8 |
| Distance between front teat | 14.2 | 14.9 | 15.5 | |
Udder conformation changes over lifetime of the animal. Moreover, selection of cows favours (directly or indirectly) survival of cows with better udder conformation. This implies, that either observations are to be adjusted for age effects, or observations used for genetic evaluation are to be taken from a specified age only. In general, (inter)national evaluations are based on observations during first lactation only.
Summary
The most complete udder health index includes direct and indirect udder health traits. An example of a direct trait is the inclusion of clinical mastitis in the index as happens in the Scandinavian countries. In some other countries, like The Netherlands, Canada and the United States, only indirect traits are used in the udder health index. These indirect traits can be subdivided in three main groups: somatic cell count, milkability and udder conformation traits.
- Recording clinical mastitis directly by a farmer or veterinarian: outer visual signs on the udder or the milk.
- Recording subclinical mastitis: not visual directly, but only perceptible by indicators. The most frequently used indicator is the number of somatic cells in milk (SCC), which can be routinely recorded parallel to milk recording.
Figure 8. Good recording practices udder health index. - Recording udder conformation. There are several udder conformation traits with an influence on udder health. The most important one by far is udder depth, followed by fore udder attachment and teat length.
- Recording milkability (i.e., milking speed) by actual measurement or (linear) appraisal by the farmer. Milkability is an optimum trait: high milking speed is favourable as it reduces labour time for milking, but it increases leakage of milk and thus bacterial invasion of the teat canal.
Decision-support for udder health recording
Reader instruction
This chapter gives a stepwise description of the possibilities to record udder health and correlated indicator traits. The starting-point is a situation in which not many efforts have been done yet, to improve udder health. In each step, a description is given on “What ?” to record, by “Who ?” this is done, and “When ? “.
Interbull recommendation animal ID
Each animal’s ID should be unique to that animal, given to the animal at birth, never be used again for any other animal, and be used throughout the life of the animal in the country of birth and also by all other countries. The following information contained in Table 14 should be provided for each animal. For further details please refer to INTERBULL bulletin no. 28 (2001).
| Table 14. Interbull recommended identification. | |
| Breed code | Character 3 |
| Country of birth code | Character 3 |
| Sex code | Character 1 |
| Animal code | Character 12 |
Interbull recommendation pedigree information
Birth date and sire and dam IDs should be recorded for all animals. Genetic evaluation centers should, in cooperation with other interested parties, keep track and report percentage of animals with missing ID and pedigree information. The overall quantitative measure of data quality should include percentage of sire and dam identified animals or alternatively percentage of missing ID's. Measures should be adopted to reduce the percentage of non-parent identified animals and missing birth information to very low numbers and ideally to zero. Examples of such measures are supervision of natural matings and artificial inseminations, avoidance of mixed semen, monitoring parturitions, comparison of birth date with calving date of dam, taking bull's ID from AI straws, etc. If there is the slightest doubt about parentage of a calf, utilization of genetic markers, e.g. micro-satellites, to ascertain parentage at birth is recommended. Until this goal is achieved, it is the INTERBULL recommendation that doubtful pedigree and birth information to be set to unknown (set parent ID to zero).
Step 0 - Prerequisites
Before an udder health system can be developed, a number of prerequisites should be accounted for:
- Unique animal identification and registration.
- Unique herd identification and registration.
- Individual animal pedigree information.
- Birth registration.
- A well functioning central database.
- Milk recording system (time information and logistics of sampling milk samples).
General definitions
A lactation period is considered to commence on the day the animal gives birth. A lactation period is considered to end the day the animal ceases to give milk (goes dry). The lactation number refers to the number of the last lactation period started by the animal. The number of days in lactation denotes the time span between calendar date of the mastitis incident and the day the last lactation period commenced. The number of days in lactation may be negative when the incident occurs during the dry-period proceeding next calving. For more detailed information on the definition of lactation period, please see ICAR guidelines Section 02.
Step 1 - Somatic cell count
What? In a milk recording system, with regular intervals milk samples are taken per cow. Samples are being gathered and taken to an official laboratory for analysis on contents of fat and protein. In addition, milk samples can be used for among others analysis of milk urea or somatic cell count.
Somatic cell count (SCC) in milk samples is obtained using Coulter Counter or Fossomatic equipment. Standardised procedures are available from the International Dairy Federation (www.idf.org). In milk of first parity cows, SCC ranges from 50.000-100.000 cells per ml from healthy udders to >1.000.000 cells per ml from udder quarters having an inflammatory infection. A current IDF standard is that subclinical mastitis is diagnosed in udders with milk having a SCC >200.000 cells per ml.
SCC can be presented either in absolute SCC or in classes based on the absolute SCC. As the distribution of absolute SCC is very skewed, generally a log-transformation is applied to a Somatic Cell Score (SCS). Other log-transformations are also used, sometimes including a correction of SCC for milk yield and effects like season and parity. SCS again can be analysed as a linear trait or used to define classes.
SCC and SCS are generally recorded on a periodical basis, especially when included in the regular milk-recording scheme. Per record, the unique animal number and day of sampling are to be supplied. When recorded on a periodical basis, animals just starting their lactation may be included. Milk in the first week of lactation has a strongly augmented level of SCC and records on animals less then 5 days in lactation are generally ignored in further analyses.
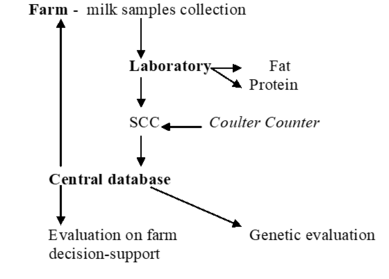
Who? Milk samples are taken either by an officer of the milk recording organisation or by the farmer. Logistics of handling samples (from the farmer to the laboratories) are generally organised by the milk recording organisation. It is important that these logistics include a strict unique identification of herd and individual cow number with each milk sample. Lab results will be transferred to the milk recording organisation, the last one also taking care of reporting the results in an informative way to the farmer.
When? Sampling of milk of individual cows for analysis of fat and protein content, and thus also for SCC, is generally done with a three-, four- or five-weeks interval. With common milking systems, twice a day, sampling includes both morning and evening milking. With automated milking systems (robotic milking), sampling can be automatically performed on a 24-hours basis, taking samples from each visit of the cow to the robot.
Step 2 - Udder conformation
What? There are several characteristics that can be measured on the conformation of the udder. The most common ones are fore udder attachment, front teat placement, teat length, udder depth, rear udder height and median suspensory ligament (ICAR Guidelines Section 05). Scoring these traits happens by scaling from 1 to 9. The figures below show the possibilities:

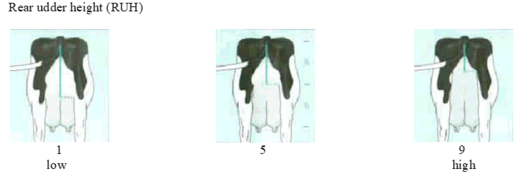
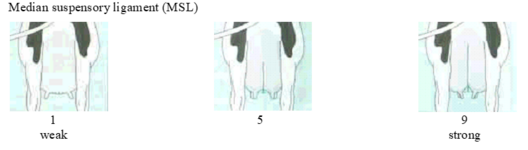
A report per cow is made of the six udder conformation traits mentioned above. An example of such a report is in Table 15 below.
| Table 15. Example of linear scoring report. | |||||||
| Inspector | Piet Paaltjes | ||||||
| Organisation | Top-cow-bred | ||||||
| Herd | Hiemstra-dairy UBN 3459678 | ||||||
| Date of inspection | May 24, 2002 | ||||||
| Cow number | Fore udder attachment | Front teat placement | Teat length | Udder depth | Rear udder height | Median suspensory ligament | |
| 154389505385 | 5 | 4 | 3 | 6 | 8 | 7 | |
| 154389505392 | 3 | 3 | 5 | 2 | 4 | 4 | |
| 154389505404 | 7 | 6 | 5 | 7 | 7 | 8 | |
| 154389505413 | 2 | 2 | 6 | 3 | 3 | 4 | |
| …. | |||||||
| ….. | |||||||
Who? Specialised inspectors score the udder conformation from the data processing organisation. Their specialism can be guaranteed through regular meetings, where new standards can come up for discussion. The WHFF organises international standardisation of inspectors for the Holstein Friesian breed. The inspectors bring the records to the data processing organisation, where the records will be processed, stored and used for evaluation. Again, it is important that the reports include a strict unique identification of herd and individual cow number. The inspectors also leave a copy of the report with the farmer.
In order to let the udder conformation information be useful for estimating udder health, linkage of the udder conformation data to the SCC-information should be warranted.
When? In most current conformation scoring systems, only the cows in their first lactation are scored. This makes scoring at least once a year necessary, assuming a calving interval of 12 months. However, it would be better to score more than once a year, for example once per 9 months. A heifer with a calving interval of 11 months will be dried off after 9 months. Such a heifer can be missed, when scoring only once per 12 months is performed.
Step 3 - Milking speed
What? The milkability (or milking speed) can be measured routinely on a large scale by subjectively scoring (the milking speed of certain small numbers of cows can be measured with advanced equipment). A milkability-form contains the individual cows together with the possibilities “very slow, slow, average, fast or very fast milking”. An example of a milkability-form is in Table 16.
| Table 16. Milkability-form example. | ||||||
| Person scoring | Farmer | |||||
| Organisation | Top-Cow-Bred | |||||
| Herd | Hiemstra-dairy UBN 3459678 | |||||
| Date of recording | May 24, 2002 | |||||
| Cow number | Very slow | Slow | Average | Fast | Very fast | |
| 154389505385 | x | |||||
| 154389505392 | x | |||||
| 154389505404 | x | |||||
| 154389505413 | x | x | ||||
| …. | ||||||
| ….. | ||||||
Who? The milkability-forms have to be filled up by the farmer. The farmer can send the form to the milk recording organisation or give the form to the officer of the milk recording organisation during the milk recording. After this the information can be used for the evaluation. Again, it is important that the forms include a strict unique identification of herd and individual cow number.
In order to let the milkability information be useful for estimating udder health, linkage of the milkability data to the SCC-information should be warranted.
When? As the milking speed does not really change over lactations, estimating the milking speed only in the cow’s first lactation is sufficient. Again, assuming a 12 months calving interval, makes a scoring of the milking speed once a year necessary.
Step 4 - Clinical mastitis incidence
What? In recording of udder health, the following general trait definition is recommended (following IDF recommendations):
- Clinical mastitis = inflammatory response of the udder: painful, red, swollen udder, with fever. This results in abnormal milk, and possibly outer visual or perceptible signs of the udder. Besides the cow can show a general illness.
- Healthy udder = absence of clinical or sub-clinical mastitis.
| Table 17. Example of form for farmers recording mastitis incidents. | |||
| Person scoring | Farmer | ||
| Organisation | Top-Cow-Bred | ||
| Herd | Hiemstra-dairy UBN 3459678 | ||
| Period of inspection | January-June, 2002 | ||
| Ear tag number cow | Date | Details | |
| 0538 | January 26 | Extremely clotted and watery “milk” | |
| 0576 | February 5 | - | |
| 0529 | April 17 | Teat injury | |
| 0541 | May 31 | Culled June 2nd | |
| 0602 | June 2 | Veterinary treatment | |
| …. | |||
Who? A veterinarian or the farmer can record clinical mastitis incidence. The obtained information has to be processed (at the farm, by the veterinary service, or e.g., the milk recording organisation) and sent to a central database, which can be done by telephone or computer either from the farm directly or from the processing organisation.
When? Except for some specific infections during the growing period, mastitis is related to the lactation of the adult female. Individual mastitis incidents are to be recorded specifying calendar date, and a database link (using a unique animal number) then will have to provide lactation number and number of days in lactation. For this purpose the database will have to include birth date and calving dates of the individual animals.
The incidence of mastitis is generally expressed per lactation period, specifying lactation period number (or parity of the cow). Standardised length of the lactation period is 305 days. However, for mastitis incidence a standardised period of 15 days prior to calving until 210 days after calving is advised (or to date of culling if less than 210 days after calving).
Clinical mastitis can be recorded on a daily basis, i.e., all (new) incidents are registered when they are (first) observed and/or when they are (first) treated. Cows having no incidents are afterwards coded ‘healthy’. Clinical mastitis can also be recorded on a periodical basis, e.g. by a veterinarian visiting the farm monthly, coding all animals momentary diseased or healthy.
Additional information on mastitis incidence may be obtained from culling reasons. Culling reason potentially makes it possible to identify cows with mastitis that are culled instead of treated. When the culling reason is mastitis, this can be considered as an additional incident.
With registration on a daily basis, it becomes feasible to define the length of the incident. However, this requires very careful observation and registration. An incident may be defined as ‘repeated’ when the observation or veterinary treatment is 3 days or longer after the former observation or treatment. Other additional information on udder health is in recording the quarter.
| Table 18. Examples of clinical mastitis specifications | |||
| Specification data | Specification definition | Reference ' | |
| Norwegian Red, first parity | Clinical mastitis (0/1) -15-210 days, including culling reasons | 20.5 % of the cows had clinical mastitis | Heringstad et al. 2001 (Livestock Production Science, 67: 265-272) |
| US Holstein Friesian, first parity | Total number of clinical episodes | On average 0.48 (sd 1.03, range 0 to 8) | Nash et al., 2000 (Journal of Dairy Science, 83: 2350‑2360) |
Summarising mastitis
Basic observation: clinical mastitis, subclinical mastitis, healthy.
To be coded as:
- Clinical vs (2) subclinical vs (0) healthy, or
- Clinical vs (0) subclinical + healthy, or
- Clinical + subclinical vs (0) healthy.
Primary data is unique cow number + observation mastitis + calendar date. This allows combination with other herd data, pedigree data, reproduction and milk recording data. This also allows calculation of a contemporary group mean (e.g., based on all animals in the same herd and parity).
Other aspects are:
- Recording of incidents per lactation period -10 to 210 days in lactation
- Repeated observation when 3 days or longer after last observation
- Inclusion of culling for mastitis as additional incident.
Other udder health information
- Bacteriological culturing of milk samples to find the specific bacterium responsible for the inflammation (e.g., Staphylococcus aureus, coliform, Streptococcus agalactiae ) - recommendations on standard methodology are provided by the IDF
- Removal of teats, teat injuries - there are standards for scoring of teat injuries, but these are not included in any official guideline
For the recording of subclinical mastitis, we can also use measurements others than SCC, either from on-line recording in the milking parlour or from centralised analysis of milk samples. In these recommendations, no further attention is paid to conductivity of milk, NAG-ase, and cytokines. A lot of work in this area is in progress and some of it is already implemented in automated milking systems - for further information we refer to information of the ICAR Recording and Sampling Devices sub-Committee.
Step 5 - Data quality
Recorded data should always be accompanied by a full description of the recording programme.
- How were herds selected?
- How were recording persons (e.g., veterinarians, and farmers) selected and instructed? Any standardised recording protocol used?
- What types of recording forms or (computer) programs are used? - What type of equipment is used?
- Is there any (change of) selection of animals within herds?
Each record should at least include a unique individual animal number, and the recording date. In case of mastitis, also a unique identification of person responsible for the recording is to be included. The unique individual animal number should facilitate a data link to a pedigree file (e.g., sire), milk recording file (e.g., calving date, birth date) and to a unique herd number. When this data links can not be established, each record on mastitis and somatic cell count should also include pedigree, birth date, calving date and parity and unique herd number.
After completion of recording, precise specification is required of any data checking, adjustment and selection steps.
Examples:
- What types of data checks are practised? (E.g., does the unique number exist for a living animal, or is recording date within a known lactation period?)
- Are averages and standard deviations within herds or per recording person standardised?
- Is a minimum of records per herd, per animal or whatever applied before data analysis is started?
Consistency and completeness of the recording and representativeness of the data is of utmost importance. Any doubt on this is to be included in a discussion on the results. The amount of information and the data structure determine the accuracy of the result; measures of this accuracy should always be provided.
For general information on data quality, we refer to Interbull bulletin no. 28, and the reports of the ICAR working group on Data Quality.
Decision-support for genetic evaluation
Genetic evaluation
Information from a single farm can be combined with information from other farms to serve as a basis for a genetic evaluation (per region, country, or breeding organisation, or even internationally). A first prerequisite is of course that information is recorded in a uniform manner. A second prerequisite is a (national) database with appropriate data logistics to combine pedigree files (herd book, identification and registration), milk recording files and files with reproductive data.
Presentation of genetic evaluations
It is recommended that breeding values on udder health for marketed sires are available on a routinely basis, i.e., included in a listing of marketed sires by official organisations. The udder health index might be considered one of the major sub-indexes. The udder health index itself should preferably be composed of predicted breeding values for direct traits and predicted breeding values for indirect, indicator traits (i.e., udder conformation, SCS and milk flow). Combination of direct and indirect information maximises accuracy of selection on resistance towards clinical and subclinical mastitis. In turn, the udder health index should be used to compose an overall performance index, for an overall ranking of animals.
The udder health index can be presented
- Either in absolute units (e.g., monetary units or % of diseased daughters) or in relative terms.
- Using either an observed or standardised standard deviation.
- Relative to either an absolute or relative genetic basis (e.g., as a deviation from 100).
It is recommended that a uniform basis of presenting indexes for functional traits is chosen per country or breeding organisation.
Within the udder health index, the weighting of predicted breeding values (PBVs) for direct and predictor traits is to be based on the information content - dependent on relationship between trait and udder health, and the accuracy of the PBVs (i.e., the number of underlying observations). As the information contents generally differ per sire, relative weighting within the udder health index should be performed on an individual sire basis.
Weighting of the udder health index as part of an overall ranking index is to be based on the relative (economic, ecological and social-cultural) value of genetically improved udder health relative to other traits.
Claw Health in Dairy Cattle
Introduction
Claw and foot disorders have become a major concern of dairy farmers around the world. They are among the major culling reasons in dairy cattle and play a significant role for the profitability of farms. Compromised animal welfare is caused by their high incidence, severity and repetitive occurrence.
Different data sources related to claw and foot disorders are available, including data from veterinarians, claw trimmers and farmers. The recording of claw health data during regular claw trimming has been identified as a particularly valuable source of information for herd claw health management and for genetic evaluation. However, integration of data for monitoring and improving dairy health should be carefully considered.
Nordic countries have pioneered the recording of claw health from claw trimming visits and then systematically using the data. Routine documentation of claw health data started in Sweden in 2003 and one year later in Finland and Norway (Johansson et al. 2011[85], Ødegård et al. 2013[86], Häggman & Juga 2013[87]). Since 2006 claw health data has been routinely recorded in the Netherlands. In several countries it is now possible to electronically register data from claw trimming visits and recording systems and consequently accessibility of claw data have improved. Electronic systems by professional trimmers to document claw health status are,for example, used in Denmark, Finland, Sweden, Norway, Canada, France, Germany, and Spain (Kofler, 2013[88]). With this development, larger amounts of claw health data are becoming available, implying the need for harmonization and further measures to strengthen data quality and consistency.
The ICAR Claw Health Atlas[89] was published in 2015 (Egger-Danner et al. 2015[90]) and has so far been translated to nineteen languages. The aim of this atlas was to harmonise the collection of high quality data within and across countries.
The purpose of these ICAR guidelines is to give recommendations on recording, data validation and use of claw health information, with focus mainly on claw trimming data.
Definitions and Terminology
A description of each of the types of data related to claw health is provided in Table 19.
| Table 19. Types of data related to claw health. | ||
| No. | Type of data | Description |
| 1 | Claw Trimming Data | Several studies have shown that data recorded by hoof trimmers are suitable for genetic evaluation of claw health (Häggman & Juga 2013[87]; Koenig et al. 2005[91]; van Pelt 2015[92]). Claw disorders are included in the comprehensive ICAR Central Health Key, that is consistent with the ICAR Standard for claw data recording and the ICAR Claw Health Atlas (see appendix of the ICAR Health guidelines). These standards should be referred to in electronic systems supposed to facilitate data recording in connection with claw trimming.
The high coverage and regular structure of the claw trimming data make them highly valuable for analyses, and these guidelines will focus on that source of information on claw health. |
| 2 | Veterinary Diagnoses | In addition to information from claw trimming, veterinary diagnoses are an additional source of information that is informative especially for more severe cases. This information is available in countries with routine recording of diagnoses, often directly in connection with veterinary interventions and medical treatments, including the Nordic countries, Austria, and Germany (Aamand, 2006[93]; Egger-Danner et al., 2012[94]; Østerås et al., 2007[95]). Analyses of claw disorders exclusively based on veterinary diagnoses are expected to have much lower frequencies than those based on hoof trimming data and may include only diseases found in lame cows. Integrated use of data, including records from regular preventive trimming, will accordingly give a more complete picture of the claw health status of the herd. More information on the collection and use of health data is available in chapter 1 (Dairy Cattle Health). |
| 3 | Lameness and locomotion scoring | Lameness describes irregularity of locomotion and can have very different causes. However, in most cases it can be seen as a sign (symptom) of a painful condition in the locomotor system and more specifically in the limbs.
This implies that the results of lameness examinations (which is the distinction between lame and non-lame animals) and data from locomotion scoring (e.g. 9-point scale used for conformation scoring – refer to Section 05 of ICAR Guidelines); 5-point-scale such as the system described by Sprecher et al., 1997) could be useful as indicators in analyses focused on claw health. There are alternative systems to be applied according to intended users and use (e.g. Sprecher et al., 1997[96]; Flower & Weary, 2006[97]). Several studies have shown that the results from screening of locomotion can be used for supporting and improving herd management and breeding (Berry et al., 2010[98]; Gaddis et al., 2014[99]; Koeck et al., 2014[100]). Although the causes of lameness or disturbed locomotion remain unclear and limits the value of working exclusively with indicator traits alone, they may become obvious when referring to incidences of individual claw health traits as measures of success. Therefore, the use of information on whether or not an animal showed clinical signs of pain and the severity can be very valuable. The results from Egger-Danner et al. (2017) [101]indicate that this information could be used for breeding purposes despite the fact that lameness scores do not identify the causes of lameness. Locomotion and lameness data are integral parts of recording systems for routine welfare assessments on farms, so increasing coverage may be expected for the future. The increased amount of data may at least partly outweigh the shortcomings of scoring systems regarding detection of early and mild cases with slightly impaired locomotion (Tomlinson et al., 2006[102]; Tadich et al., 2010[103]; Bilcalho & Oikonomou, 2013[104]). |
| 4 | Feet and Legs conformation traits | Type traits associated with feet and legs are included as part of the conformation assessment of breed societies and dairy cattle breeding organisations and as such are also covered by Section 05 of the ICAR guidelines. Data from this routine and internationally harmonized way of collecting data may be considered as source of additional information for claw health improvement.
Studies in different countries and breeds have revealed conflicting results regarding the correlations between conformation of feet and legs on the one hand and claw health on the other hand: There are only a few reports showing favourable correlations (Fuerst-Waltl et al., 2015; van der Linde et al., 2010) while most studies have weak correlations and consequently limits the use of conformation traits as indicators (e.g., Koenig and Swalve, 2006; Häggman and Juga, 2013; Ødegård et al., 2014). However, locomotion assessment is an exception and showed more consistent results and moderate correlations, although scored only in non-lame cows and usually only once in first parity cows. |
| 5 | Data from Automation | Different systems are becoming available for automated recording of data on activity, locomotion pattern, lying and feeding behaviour of cattle, including pedometers, video image analysis, thermography and other sensors. Although the focus of their use is often oestrus detection, these measurements can provide useful information for early and more accurate detection of lameness and foot pathologies (Alsaaod et al., 2015[105]; Beer et al., 2016[106]; Nechanitzky et al., 2016[107]). Experiences with broader use of this type of data, which is becoming increasingly abundant is still limited; but parameters such as number and duration of lying bouts, number and length of strides, walking speed, bite rate while grazing, duration and pattern of feed intake and rumination have been shown to be different between healthy and sick cows (Beer et al., 2016[108]). Their potential to help identify animals that require special health care within farms is likely to be increasingly exploited, and routines for using automated data across herds in the context of claw health improvement are expected. |
Definitions of claw health disorders according ICAR Claw Health Key
To be able to combine and compare claw health data between countries and for breeding purposes, standardizing the recording and harmonizing the terminology of claw disorders are crucial. Harmonized definitions have been published by the ICAR WGFT (Egger-Danner et al., 2015[109]). The Atlas describes 27 claw disorders (Table 20); the corresponding ICAR Claw Health Atlas illustrates the distinct disorders by typical pictures in a number of languages.
| Table 20. Abbreviations and harmonized descriptions of foot and claw disorders (Egger-Danner et al., 2015[109]). | |||
| Name | Code | Description | Synonymous Terms |
| Asymmetric claws | AC | Significant difference in width, height and/or length between outer and inner claw which cannot be balanced by trimming | – |
| Corkscrew claw | CC | Any torsion of either the outer or inner claw. The dorsal edge of the wall deviates from a straight line | – |
| Concave dorsal wall | CD | Concave shape of the dorsal wall | – |
| Digital dermatitis | DD | Infection of the digital and/or interdigital skin with erosion, mostly painful ulcerations and/or chronic hyperkeratosis/proliferation | Mortellaro disease, Strawberry disease |
| Interdigital/
superficial dermatitis |
ID | All kind of mild dermatitis around the claws that is not classified as digital dermatitis. | – |
| Double sole | DS | Two or more layers of under-run sole horn | Underrun sole |
| Heel horn erosion | HHE | Erosion of the bulbs, in severe cases typically V-shaped, possibly extending to the corium | Slurry heel, Erosio ungulae |
| Horn fissure | HF | Crack in the claw wall | – |
| Axial horn fissure | HFA | Vertical (longitudinal) crack in the inner claw wall | – |
| Horizontal horn fissure | HFH | Horizontal crack in the claw wall | – |
| Vertical horn fissure | HFV | Vertical (longitudinal) crack in the outer or dorsal claw wall | – |
| Interdigital hyperplasia | IH | Interdigital growth of fibrous tissue | Corns, Tyloma, Interdigital fibroma |
| Interdigital phlegmon | IP | Symmetric painful swelling of the foot commonly accompanied with odorous smell with sudden onset of lameness | Foot rot, Foul in the foot, Interdigital necrobacillosis |
| Scissor claws | SC | Tip of toes crossing each other | – |
| Sole hemorrhage | SH | Diffused and/or circumscribed red or yellow discoloration of the sole and/or white line | Sole bruising |
| Sole hemorrhage diffused form | SHD | Diffused light red to yellowish discoloration | – |
| Sole hemorrhage circumscribed form | SHC | Clear differentiation between discoloured and normal coloured horn | – |
| Swelling of coronet and/or bulb | SW | Uni- or bilateral swelling of tissue above horn capsule, which may be caused by different conditions | – |
| Ulcer | U | Ulceration of the sole area specified according to localization (zones) such as bulb ulcer, sole ulcer, toe ulcer/necrosis | – |
| Sole ulcer | SU | Penetration through the sole horn exposing fresh or necrotic corium. | – |
| Bulb ulcer | BU | Ulcer located at the bulb | Heel ulcer |
| Toe ulcer | TU | Ulcer located at the toe | – |
| Toe necrosis | TN | Necrosis of the tip of the toe with affection of bone tissue | – |
| Thin sole | TS | Sole horn yields (feels spongy) when finger pressure is applied | – |
| White line disease | WL | Separation of the white line with or without purulent exudation | – |
| White line abscess | WLA | Necro-purulent inflammation of the corium | – |
| White line fissure | WLF | Separation of the white line which remains after balancing both soles | – |
The most common classification of claw disorders makes the distinction between infectious and non-infectious disorders (Alsaood et al., 2015). Infectious disorders are primarily digital dermatitis, interdigital dermatitis, interdigital phlegmon, and heel horn erosion. Non-infectious disorders include claw horn disruptions (also called claw horn disorders), sole hemorrhages, white line fissure, horn fissures, ulcers, thin sole, and all kinds of claw distortion. However, several disorders that affect the claw horn capsule, such as wall, sole, and its junction, i.e. white line, are often secondarily infected. This also applies to interdigital hyperplasia which is usually considered to be non-infectious, too, although pathogenesis is still partly unknown.
Definitions of other terms used in these guidelines
Definitions of Terms used in these guidelines are given in Table 21.
| Table 21. Definitions of terms used in these guidelines (detailed information is found in chapters 0 and 4.6). | |
| Term | Definition |
| New lesion | A claw disorder recorded for the first time in a particular location or claw or recoded later than the minimum recovery period after the previous recording of the same kind in the same location or claw. |
| Chronic cow and persistent lesion | A chronic cow is a cow presenting a persistent lesion over a prolonged period and/or several relapses such that shows the same disorder after 3 consecutive trimmings during lactation, with intervals in between exceeding the period of time previously established and required to define a new lesion. |
| Incidence rate | The proportion of cows developing at least one new case of a claw disorder relative to all cows screened for claw disorders with comparable density in a certain period of time (e.g. annual incidence rate). |
| Prevalence rate | The proportion of cows affected by a particular claw disorder relative to all cows screened for claw disorders in a certain period of time or at a certain point of time (e.g. annual prevalence rate, trimming visit prevalence rate). |
| Cows at risk | Cows screened for presence of claw disorders, so cows presented for trimming at a particular date or cows present in the herd and included in regular checking of claws. |
| Time period at risk | Time frame defined for benchmarks (e.g. year, season or lactation period). |
| Reference levels | Figure defined for benchmarking which specification by, e.g. herd size, production level, geographic location, flooring, housing systems, trimming policy, season, parity, age and stage of lactation. |
Scope
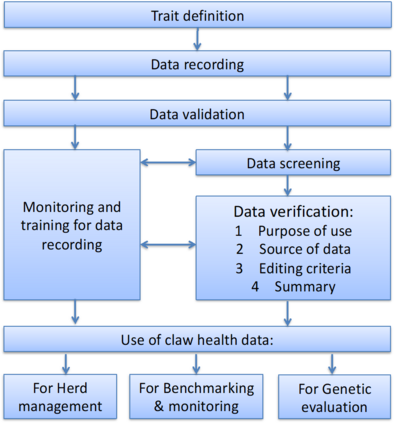
Figure 10 gives a summary of the main elements of this guideline. The current guidelines on claw health cover only data recorded by hoof trimmer.
Trait definition - claw trimming data
More detailed information is available under Egger-Danner et al. 2015[109], Christen et al. 2015[110] and here on the ICAR website.
Definition - claw trimming data
At trimming the claw health status of each cow is recorded. Cows with no claw disorder should be recorded as healthy, and presence of any defined claw disorder (Table 20) should be recorded at animal, leg or claw level.
The number of records and the level of specific details used vary between recording systems (see codes Table 20). Traits can be defined more in detail if additional information on location (e.g leg/claw/position) and severity is recorded (refer chapter 4.5 - Data Recording – claw trimming data).
New lesion
For a specific disorder, the differentiation between a new episode, or a new lesion and a previous case requires a definition of the recovery period of each lesion (if possible). For some disorders (AC CC CD and SC) the process is permanent or irreversible, so no healing period can be defined. For other claw disorders a recovery period of 4 months can be used, i.e. if a new case is recorded more than 4 months after the previous case it can be assumed to be a new lesion. On the other hand, the development of the same lesion (e.g. WLD) on another location (claw) is considered to be a new lesion.
Chronic cow and persistent lesion
A chronic cow is a cow which shows a persistent lesion over a long period and/or shows various relapses during lactation. It could be due to a failed treatment or to a delay in recognition. In order to differentiate an acute lesion from a chronic one, it is important to know the period of time that has passed since it first appeared, or the number of relapses recorded for the same lesion. This is a key concept when it comes to make decisions about individual cow in terms of herd management. A chronic claw health lesion is defined as a lesion which persists over 3 consecutive trimmings during lactation, with intervals in between exceeding the period of time previously established and required to define a new lesion.
Data Recording – claw trimming data
The conditions and circumstances of claw health management differ widely across countries (Christen et al. 2015[110]). The percentage of trimmings recorded by professional trimmers varies. Claw care is generally carried out by trained farm staff, professional claw trimmers, or the farmers themselves. Different tools are used to record information on claw disorders and foot and leg conditions, including individual free-text notes (no standardized form), standard forms with reference to the key for claw health on paper sheet reports, free-text or standard forms on mobile electronic devices, and herd management software. For use in routine genetic evaluations for claw health, data from claw trimming need to be recorded routinely and stored in a central database. For advanced herd management tools with benchmarking and comparison between farms, central data storage is necessary as well. A key aspect of the successful initiatives to build routine genetic evaluations for claw and leg health is the development of an infrastructure for electronic documentation and recording of claw trimming data (Kofler et al., 2011[110], 2013[111]; Nielsen, 2014[112]; Van Pelt, 2015[113]). Data security aspects have to be given special attention and measures have to be implemented around the transparency of use of data and protection of personnel.
Minimum requirements:
- Animal-ID
- Herd-ID
- Records on animal level
- Date of trimming
Highly recommended:
- Trimmer-ID (it is essential for data validation but also very valuable for the use of the data)
Optional/additional information:
- Recording the location of the disorder/lesion: leg (e.g. left front leg), claw (inner or outer claw), positions (claw zones (Kofler et al. 2011[114]))
- Recording of severity degree: e.g. mild, severe, M-stages for DD (Dopfer, 2009[115]).
Data Validation
The validation of data is based on a comparison between collected data and valid references to ensure that data is compliant with standards and fit for the intended use. The challenge with the validation process is to choose appropriate criteria and adequate levels in order to extract reliable information from raw data. There are two main steps in the data validation process: data screening and data verification.
Data Screening
Data screening consists of a series of basic checks on integrity, format and completeness. For instance, checks can be made on ID plausibility for animals, herds and diagnosis codes, which are necessary to avoid suspect values. Other checks can be on the plausibility of dates, verifying dates of birth, calving and diagnosis in order to eliminate typing errors. Data screening is usually implemented as data filters, routines or algorithms applied when entering data (included as default in pc-tablet applications or when new data is uploaded to the central database) or manually when new data is added to an existing claw database.
Check for data screening include:
- valid animal-ID
- valid claw disorder code
- valid date
- valid herd – ID (animal assigned at date of claw disorder to farm)
- additional criteria for more optional recorded information (e.g. severity grades within range)
Data Verification
Data verification consists of checking the correctness of data. Completeness of data recording on farm should be considered as well. The exhaustiveness and the completeness of the process depends on the purpose of use and on the data sources:
Purpose of use
Depending upon the intended use, the quantity and quality of data is important, in relation to the purpose. At the farm level the farmer, or the trimmer/vet, will use the recorded data to manage cow-level decisions and to evaluate current claw health and to get an insight into causes of possible claw-health and lameness problems. Moreover, it is used to assess the effect of previous management measures, to take decisions on herd management and to understand the reasons of fluctuations of claw health status when they occur. Another use is for benchmarking analysis in order to define benchmarks and standards that serve as references for evaluating claw health status. Claw data are also used in genetic analyses, to estimate breeding values and genetic trends.
Herd management analysis requires as much complete data as possible, and should include as much information as possible about the risk factors. Therefore, this type of validation is usually less restrictive since it mainly checks the completeness of the data. If the data are used by the farmer, a basic data check is done on farm.
When it comes to data for research and routine genetic evaluation, data validation needs to be more exhaustive in order to use only information from farms that can be considered as reliable. The data editing process is usually more exhaustive in order to ensure data correctness.
For benchmarks, calculation and monitoring, data must be checked for representativeness. Information on herd size, housing system, and geographic location should be taken into account to ensure the data are representative. Herds with outlier parameters should be eliminated. The percentage of trimmed cows within herds must be as high as possible. Benchmarks are often calculated without considering environmental effects in the model. For interpretation and comparability of benchmarks environmental information included as well as information on calculation and data validation have to be considered as these might have a big impact on the results.
Source of data
The origin of data has an impact on the reference levels used to check data quality. Depending on the recording system, claw health data are recorded by trimmers, veterinarians and/or farmers. A large proportion of data is usually provided by trained trimmers who register claw health data during preventative trimming or treatments, while veterinarians generally register only the most severe cases. Thus, the majority of claw health data are recorded either by claw trimmers or herd staff and not by veterinarians. Therefore, the data provided by trimmers, or collected by farmers usually show a higher incidence rate than the data supplied by veterinarian. The diagnoses of veterinarians and claw trimmers, however, may be more accurate than those of farmers. The routine collection of information via claw trimmers may provide a much more reliable picture on the prevalence of claw disorders in dairy cattle. In most cases, we have to deal with a combination of data from different sources.
Editing criteria
In order to ensure the correctness and the accuracy of the data, several editing criteria have been reported within each level of data.
Trimmer/Vet data verification
In general, data on claw disorders are collected by hoof trimmers during scheduled (mainly), or emergency visits. A minimum number of records should be required per trimmer to ensure continuity and representativeness of the collected data (Perez-Cabal & Charfeddine, 2015[116]). Data recorded in training periods should be removed. Besides, incidence rate for each disorder could be calculated and compared with the overall incidence rate of other trimmers (in the same area/country and time period) and checked whether it is within the range of e.g. two standard deviations (to ensure uniformity in recording and to detect under- or over-reporting).
Recommendation
- minimum number of records per trimmer
- check for continuity of data provision from trimmer
- calculate incidence rates and variation per trimmer – see also 4.6.3 Monitoring and training for data recording.
- check plausibility if data are generated by different persons
Herd level verification
Routines for claw trimming may vary, but trimming is often done once or twice a year for each cow. Typically, the farmer selects the cows to be trimmed, that is why a minimum number of records per herd and per year and a minimum percentage of present cows trimmed per herd and year are required in order to avoid selection bias (e.g. Van der Spek et al., 2013[117]). For herd management, the percentage of cows trimmed should be used to establish the reference group for comparisons within herd. Depending on the use of data, a minimum frequency could be required to avoid using data from herds that under-report (mainly used for genetic analysis and benchmarking calculation). Additional checks on herd-trimming days are used to ensure that a minimum percentage of present cows are trimmed and there is a minimum number of animals without disorder per visit (e.g. van der Waaij et al., 2005[118]). Because herd sizes, data structure and management practices vary among countries, the level of minimum incidence rate or the number/percentage of trimmed cows that are required needs to be defined accordingly to avoid a massive elimination of useful data.
Recommendation
- check whether only trimmed cows are recorded
- minimum incidence rate for a specific disorder or for overall disorders
- minimum percentage of trimmed cows in herd in observation period
- continuity of data provision from herd
- note the strategy of trimming
Animal data verification
Checks at animal level are focused on verifying unique identification, herd location at trimming, age at calving, sire of the cow, days in milk and parity status. Claw disorders may be recorded for each claw. Moreover, in some recording protocols they differentiate between inner and outer claw. In some countries, claw disorder trait is defined at claw level, while in others the trait is defined at animal level and the score assigned to each animal is the highest value in case that the cow shows the same disorder on different claws.
Recommendation
- correct animal-ID (see screening)
- check for correct additional information (see chapter recording and trait definition)
Record verification
A claw disorder record describes the status of the claw at any given day. To validate a new record, we need to answer to the question whether this record defines a new episode with the same diagnosis or is a just a control of the same case. The time intervals used to define the following diagnosis as a new event for each disorder in the same claw is 4 months.
Recommendation
- check for new lesion or new case (see chapter 0)
Summary
Minimum criteria for validation for use in herd management:
- screening requirements
Additional recommended criteria for use for genetic evaluation:
- only valid herds (e.g. minimum % of trimmed cows)
- valid observation period (e.g. with continuous data recording; minimum % of cows with disorders)
- valid trimmers (e.g. continuous data provision; minimum amount of data within period; optional additional criteria)
Additional recommended criteria for benchmarking: define criteria depending on the reference level (e.g. herd size, breed, management system, etc.).
- Herds included should have a high percentage of cows presented at trimming.
Monitoring and training for data recording
Data collectors, which can be trimmers, veterinarian or farmers, should be reliable and accurate in order to reflect a stable and consistent collection process across persons and over time. Data collector should apply the same disorder, the same definition and scoring scale. Therefore, having a good documentation process, training course and statistical monitoring are useful to ensure a good harmonization between data collectors.
The ICAR claw health atlas should be made available to all collectors, or at least a local guideline, which should contain pictures and definitions of the disorders based on ICAR claw health atlas definitions. Also, the used scale to score the disorders of different severity degrees should be made clear in this documentation.
Regular training sessions should be made to train data collectors and to discuss different recording interpretations. A comparison between experienced persons and new ones during practical sessions could be a good way to unify criteria. Moreover, ensuring consistency between data collectors should be done by checking data collectors criteria using pictures for different disorders with varying degrees of severity and are also considered very useful to reduce variability.
Statistical analysis of data collected by each data collector, such as a calculation of the frequency of each disorder and its deviations with the rest of group, could be useful to detect under-reporting or misunderstanding of the scoring scale. In case a disorder has more than two classes, the frequency of the scores can be compared between one person and the rest of a group. More detailed monitoring per person could be done by analysing the scores per lactation number of the cow. In case a large number of scores per data collector is available, is to compute the correlation between the scores of one data collector and the scores of rest of the group by using bivariate genetic analysis. This shows the quality of harmonisation of trait definition between data collectors (Veerkamp et al. 2002[119]).
For this analysis, two data sets are created, one with scores of one data collector and the other with scores of all other data collectors from a certain period, for example 12 months. Both data sets can be analysed in a bivariate analysis, estimating different (genetic) parameters. The analysis can be carried out for each trait and for each data collector. Incidence rates per trimmer as well as from the bivariate analyses the heritability and genetic correlation can be used as indicators for data quality.
Recommendation
- Frequencies/ incidence rates per trimmer.
- Heritability: the heritability estimated within each data collector can be used as criteria for the repeatability of scores within data collectors, albeit the optimum value is not unity but depends on the true heritability of each disorder.
- Genetic correlation: the genetic correlation between two data sets can be used as a measure of the repeatability between data collectors, where a genetic correlation of one between data collectors is expected.
Use of Claw Health Data – general
Data on the claw health status of each cow provides an important insight into the health status of the entire herd and population. Benchmark parameters like incidence and prevalence rates are used to monitor the degree of claw lesions within dairy herds and to highlight the full scale of claw health problems in the whole population. The values of such parameters depend on the frequency and the recovery period of each claw disorder, which are affected by cow and herd-related risk factors. The assessment of these risk factors helps to address why rates fluctuate within herds and how to fix them.
Risk factors
Many risk factors predisposing the occurrence of claw disorders have been reported in the literature. These risk factors can be related to herd management conditions or to the individual cow status (see Annex 1: Risk factors for claw disorders).
For optimization of herd management as well as interpretation of benchmarks information related to risk factors is valuable. Targeted strategies to reduce the incidence of feet and legs disorders can be elaborated if this information is available.
Indicators/parameters for claw health
Incidence rate (IR)
Incidence rate describes the development of new cases of claw disorder. It is defined as the number of new cases of a specific claw disorder per unit of animal-time during a given time period. Incidence rate highlights the speed at which new cases of a disorder occur in the herd and therefore is more suited to assess claw health management policy.
Equation 5. Computation of incidence rate for claw health disorders.
Prevalence rate (PR)
Prevalence rate describes the percentage of cows having a claw disorder. It is defined as a proportion of cows affected by a disorder at a particular time point or during a specified time period. Prevalence takes into account the new and the pre-existing cases whereas incidence includes only the new cases. It provides an appropriate snapshot to show the magnitude of the spread of a disorder within a given population at a certain point of time (point prevalence) or during a period of time (period prevalence). Prevalence rates calculated in different countries or studies to be comparable should be calculated in the same way and for the same production system (see Annex 2: Prevalence rates for claw disorders for different breeds in several countries)
Equation 6. Computation of prevalence rate for claw health disorders.
Definitions for parameters calculation:
For the calculation of incidence and prevalence rates three important concepts should be defined:
a. Reference levels
A key point for between the herds benchmarking process is how to compare with the appropriate benchmarking group and how to establish a target related to this group. For that reason, it is important to define a comparable reference level. Reference level could be defined by herd size, production level, geographic location, flooring and housing systems, season, parity, age and stage of lactation.
b. Cows at risk
One of the challenges of a benchmark calculation is the definition of the denominator. By definition it should be equal to the number of cows at risk in the time period. However, the concept of “cows at risk during the time period” may be inaccurate if not all cows are trimmed or checked. So, if we consider cows at risk as cows present in the herd at any moment of the time period that means that non-trimmed cows are assumed to be “healthy cows”. While if we consider cows at risk as trimmed cows during the time period, then the calculated rates depend on the percentage of trimmed cows. In situations of regular lameness screening (every 1-4 weeks) then this assumption may be valid. Detection may also be influenced by the timing of the foot inspection, with lesion detection rates higher at 60-120 days into lactation in most herds. The other critical point is that we deal with open herds where animals are leaving and entering the herd throughout the time period. Dohoo et al. (2009)[120] reported that animals for which there is a loss of follow-up during the time period are called withdrawals and the simplest way of dealing with them is to subtract half the number of withdrawals from the population at risk. However, calculating animal-days within the herd is perhaps the most precise way to account for withdrawals.
c. Time period at risk
Benchmark calculation should be performed on a reference period of time which allows a fair comparison within and across herds with different management systems and at different times of the year. The time period could be defined as a year, season or lactation period.
Use of claw trimming data for herd management
Herd management is a continuous process which involves decision making and supervision of claw health status. This process starts with recording all useful data that makes claw health monitoring feasible. Documentation on claw disorders allows farmers/hoof trimmers/ veterinarians to get an up-to-date report on claw health status at herd and animal levels. Trends of prevalence rate and incidence rate within the herd and comparison with reference levels should serve as a monitoring tool for claw health. If a value is determined to be out of the desired range, an assessment of the associated risk factors should be made to allow for the implementation of corrective actions. Claw health data for herd management has a use at two different levels.
At the cow level, documentation provides data about individual cow history and allows follow-up of the healing process and re-check requirements. At the herd level documentation provides data about timing during lactation/season of hoof trimming for maintenance and lesions.
Data from claw reports should answer the following questions:
- Whether the claw health status has changed or not?
- The timing (lactation/season) of the change?
- Which cows are affected?
- Whether the farms stated hoof trimming goals are being met?
- Is the claw health strategy/new treatment working?
Figure 13 and Figure 14 show examples of graphs which can help to answer those questions at herd level.
Claw disorders are often recurrent, and there are frequently several registers for the same disorder recorded on the same claw on different dates. When using claw health data for herd management, it is important to know whether the new register defines a new disease process for the same kind of lesion or is just a control for the same episode. Moreover, it is useful to define the concept of chronic cow or chronic lesion in order to take the optimum disposal decision. Cramer & Guard (2011)[121] recommend the definition of both concepts at the level of cow’s lactation instead of at the claw’s lesion level because claw disorders on different limbs are not really independent and unless we follow very closely we cannot be sure that different records at different moments of lactation are due to different disease processes.
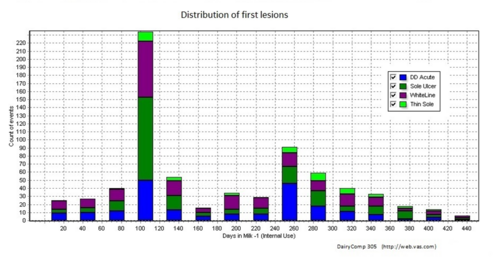
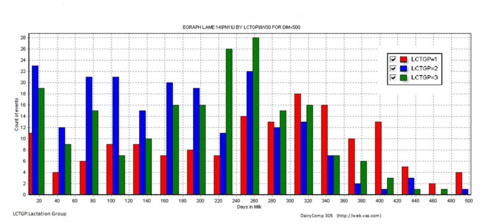
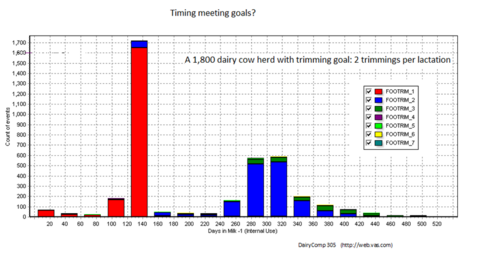
Figure 15 and Figure 16 show the list of not trimmed cows and cows showing lesions in the last three trimmings, respectively.
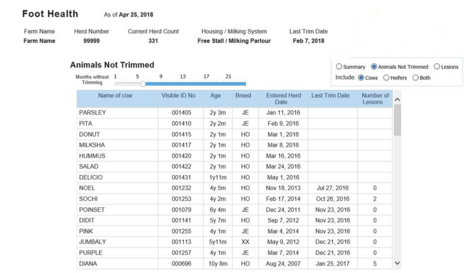
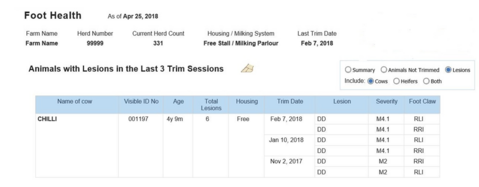
Use of claw trimming data for benchmarking and monitoring
Benchmarking is a useful tool to compare performance and the need for improvement (Von Keyserlingk et al., 2012[122]; Bradley et al., 2013[123]). Besides, it also helps to illustrate the potential benefits that improvements might offer; it can also motivate producers to adopt preventive practices and to foster the documentation of claw data. The success of any benchmarking process depends on the use of appropriate benchmarks. Incidence and prevalence rates are key parameters that can be used to make comparisons among and within herds over time (Dohoo et al., 2009[124]).
Claw health data should be able to answer the following questions:
- What is the current status?
- Does the situation change and do I need to investigate further?
- Which age group and which lactation stage are affected?
- What is the gap between the current situation and the reference level?
A useful benchmarking report should be straightforward and concise, supported by clear and informative tables and charts showing a snapshot or a trend of incidence or prevalence rate. Figures as pie chart, bar chart and/or radial chart provide a graphical assessment of claw health status. Figure 17 and Figure 18 show examples of the Canadian DHI foot health benchmark report. Figure 17 displays the frequency of claw disorders within 12-month period and compare it with different benchmarks calculated for different group of animals (heifers, cows) and three different combinations of production systems (Free-stalls with robot, Freestalls with milking parlour, and Tie-stalls). Figure 18 displays a table with healthy/lesion count for each month and throughout the year at the herd, provincial, and national levels. The colored block indicates the range of the herd's percentile rank.
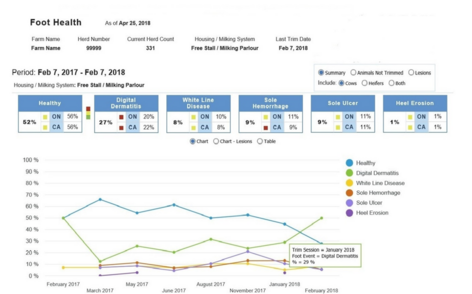
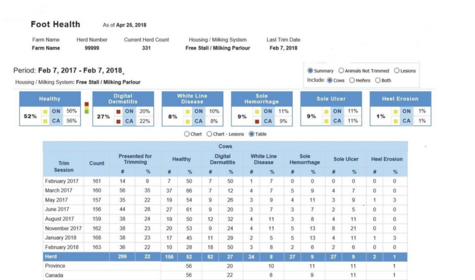
Use of claw trimming data for genetic evaluation
Routine recording of claw health status at claw trimming provide valuable data for genetic evaluations. This section covers issues related to genetic evaluation of claw health, such as data sources, trait definitions, models and genetic parameters. For more detailed information we refer to the review paper by Heringstad & Egger-Danner et al. (2018)[125].
Data sources
Different sources of data and traits can be used to describe and evaluate claw health. The most reliable and comprehensive information is data from claw trimming, and use of these data is the scope of the guidelines. Possible indicator traits include veterinary diagnoses, data from lameness and locomotion scoring, activity-related information from sensors, and feet and legs conformation traits. Indicators may be useful in genetic evaluations, but this is not discussed here.
Trait definition
Claw disorders are usually defined as binary traits, based on whether or not the claw disorder was present (recorded) at least once during a defined time period (opportunity period), usually from calving to day 305 or end of lactation.
Binary coding can be based on single specific disorders (i.e. each diagnosis is one trait) or groups or composite traits. Traits can be grouped according to aetiology and pathogenesis, e.g. infectious and non-infectious disorders, or grouping of all diagnoses as any (all) disorder. Grouping is often chosen in situations with limited data and/or low frequency of single disorders. If linear models are used the heritability will be higher for group traits than for the specific disorders as a result of higher frequency. Grouping might make comparisons for use in international evaluations difficult. Harmonized descriptions of individual disorders are important.
Alternatively, to take multiple occurrences into account can claw disorders be defined as the number of cases during a defined period time. This requires a clear definition of new cases. Also recording at the level of individual legs may be needed to accurately define new cases.
Claw health records from different parities can be treated as repeated measures of the same trait or as multiple traits. High genetic correlations justify treating claw disorders as the same trait across parities. There is a wide range of estimated correlation in the literature (e.g. van der Linde et al. 2010; van der Spek et al 2015)[126] so this should be checked in each case. Similarly, there is a question on whether the same disease occurring at different stages at lactation (e.g. early-, mid- and late lactation) should be assumed to be the same trait.
Which animals to define as cows with no claw disorders present (i.e. healthy herd mates) may be challenging as herd trimming strategies and recording practices vary. Ideally should all cows in a herd be trimmed and status of all cows, including those with normal/healthy claws, should be recorded at trimming. In most cases not all the cows be trimmed and there is a question whether non-trimmed cows should be included as healthy herd mates or excluded from the genetic analyses. Assuming that all non-trimmed cows are healthy underestimates the incidence of claw disorders (mild cases could be present, but not detected), while including only trimmed cows may overestimate the incidence (non-trimmed cows are more likely to be unaffected).
Key issues related to trait definition:
- Binary trait or number of cases?
- Single specific disorders or groups/composite traits?
- Length of opportunity period?
- Same trait across parities?
- Same trait across stage of lactation?
- Include or exclude non-trimmed cows?
Models
Effects to consider in models for genetic evaluations of claw heath, in addition to standard effects such as age, contemporary group, and lactation number, include effects of time (lactation stage) at trimming and trimmer. The latter requires that a unique ID is recorded for each trimmer. Lactation stage at trimming can be the number of days or weeks between calving and trimming. The timing of the occurrence of disease probably is less accurate when based on claw trimming rather than veterinary treatment data. Depending on the herd’s claw-trimming routine there may be some time between the occurrence of a problem and the trimming day, and milder cases may go unnoticed until trimming.
The considerations regarding choice of model for genetic evaluation for claw health will be the same as for other categorical traits. Although more advanced models may be advantageous as they utilize more of the available information, linear models may often be the model of choice for routine genetic evaluation as they are fast, easy to implement, and gives in most cases very similar ranking of animals as more advanced models.
Genetic parameters
Heritability of the most commonly analysed claw disorders based on data from routine claw trimming were in general low (Table 22[1]), with linear model estimates ranging from 0.01 to 0.14 and threshold model estimates ranging from 0.06 to 0.39. For the composite trait overall claw health (any lesion) estimated heritability varied from 0.05 to 0.07 from linear model, and from 0.07 to 0.13 from threshold model.
| Table 22. Range of heritability estimates for the most common claw disorders | ||
| Trait | Threshold model | Linear model |
| Digital / interdigital dermatitis | 0.09 - 0.20 | 0.01 - 0.11 |
| Heel horn erosion | 0.09 | 0.03 - 0.07 |
| Interdigital hyperplasia | 0.19 - 0.39 | 0.01 - 0.14 |
| Sole hemorrage | 0.07 - 0.09 | 0.02 - 0.08 |
| Sole ulcer | 0.07 - 0.18 | 0.01 - 0.12 |
| White line disease | 0.06 - 0.10 | 0.01 - 0.09 |
Estimated genetic correlations among claw disorders varied from -0.40 to 0.98 (Table 23[2]). The strongest genetic correlations were found among sole hemorrhage (SH), sole ulcer (SU), and white line disease (WL), and between digital/interdigital dermatitis (DD/ID) and heel horn erosion (HHE). Genetic correlations between DD/ID and HHE on the one hand and SH, SU, or WL on the other hand were low in most cases.
| Table 23. Range of genetic correlation estimates among digital and/or interdigital dermatitis (DD/ID), heel horn erosion (HHE), interdigital hyperplasia (IH), sole hemorrhage (SH), sole ulcer (SU), and white line disease (WL) (from Heringstad et al, 2018[127]) | |||||
| HHE | IH | SH | SU | WL | |
| DD/ID | 0.58 - 0.87 | 0.10 - 0.66 | -0.15 - 0.12 | -0.19 - 0.56 | -0.33 - 0.08 |
| HHE | -0.07 - 0.23 | -0.05 - 0.50 | 0.22 - 0.36 | ||
| IH | -0.40 - 0.13 | -0.08 - 0.50 | -0.35 - 0.34 | ||
| SH | 0.38 - 0.90 | 0.10 - 0.62 | |||
| SU | 0.01 - 0.98 | ||||
Implications
Genetic improvement of claw health is possible. However, the traits show low heritability and large scale routine recording is needed for reliable genetic evaluations. The genetic correlations to indicator traits like feet and leg conformation is low so direct selection based on genetic evaluation based on trimming data will be most efficient. As comprehensive recording of hoof trimming data is challenging it is recommended to use other direct or indirect information for genetic evaluation as well as for herd management.
Summary Check List
These guidelines provide recommendations on recording, validation, monitoring and use of claw health data.
Data Recording
For data recording the minimum requirements should be:
- Animal-ID
- Herd-ID
- Records on animal level
- Date of trimming
Trimmer-ID is highly recommended but not compulsory (it is essential for data validation but also very valuable for the use of the data). Other additional information could be useful as:
- Recording the location of the disorder/lesion: leg (e.g. left front leg), claw (inner or outer claw), positions (claw zones)
- Recording of severity degree: e.g. mild, severe, M-stages for DD
1.2.2 Data Validation
For data validation two steps have been defined: data screening and data verification.
Before data entry in the database, the information should be screened in order to ensure completeness and correctness of the data. The check should include:
- Valid animal-ID
- Valid claw disorder code
- Valid date
- Valid herd – ID (animal assigned at date of claw disorder to farm)
- Additional criteria for more optional recorded information (e.g. severity grades within range)
Before conducting further analyses, data must be verified in order to ensure that the data is fitted for the intended use. That is why the check depends on the purpose of use and on the data sources.
Genetic Analysis
For genetic analyses several editing criteria have been reported within each level of data.
At trimmer level:
- Minimum no of records per trimmer
- Check for continuity of data provision from trimmer
- Calculate incidence rates and variation per trimmer – see also training of hoof trimmers
- Check plausibility if data are generated by different persons
At herd level:
- Check for valid herds (e.g. minimum % of trimmed cows)
At animal level:
- Correct animal-ID (see screening)
- Check for correct additional information
At record level:
- Check for new lesion or new case
Benchmark
For benchmarks calculation editing criteria depending on the reference level (e.g. herd size, breed, management system, etc.) should be defined.
- Herds included should have a high percentage of cows presented at trimming.
- Valid observation period (e.g. with continuous data recording; minimum % of cows with disorders)
- Valid trimmers (e.g. continuous data provision; minimum amount of data within period; optional additional criteria)
Monitoring and Training
Monitoring and training process for data collectors is highly recommended in order to achieve a consistent collection process across persons and over time. Statistical analysis should include the calculation of:
- Frequencies/ incidence rates per trimmer.
- Heritability: the heritability estimated within each data collector can be used as criteria for the repeatability of scores within data collectors, albeit the optimum value is not unity but depends on the true heritability of each disorder.
- Genetic correlation: the genetic correlation between two data sets can be used as a measure of the repeatability between data collectors, where a genetic correlation of one between data collectors is expected.
Use of claw health data
Data on the claw health status at cow or claw level are used for herd management, benchmarking and genetic analyses.
For herd management data from claw reports should answer the following questions:
- Whether the claw health status has changed or not?
- The timing (lactation/season) of the change?
- Which cows are affected?
- Whether the farms stated hoof trimming goals are being met?
Benchmarking is a useful tool which success depends on the use of appropriate key parameters and reference levels. Benchmarking reports should be able to answer the following questions:
- What is the current performance?
- What is the position within the reference group?
Genetic improvement of claw health is possible even though claw disorder traits show low heritability. A large scale routine recording system for claw trimming data is highly needed for reliable genetic evaluations.
Acknowledgements
This document is the result of the work of the ICAR working group on functional traits (ICAR WGFT) together with internationally recognised claw experts. The members of the ICAR WGFT are, in alphabetical order:
- Andrew John Bradley, Quality Milk Management Services, United Kingdom; andrew.bradley@qmms.co.uk
- Noureddine Charfeddine (Conafe, Spain) nouredine.charfeddine@conafe.com
- John B. Cole, Animal Improvement Programs Laboratory, USA; John.Cole@ARS.USDA.GOV
- Christa Egger-Danner, ZuchtData EDV-Dienstleistungen GmbH, Austria; egger-danner@zuchtdata.at (chairperson)
- Nicolas Gengler, Gembloux Agro-Bio Tech, University of Liège, Belgium; nicolas.gengler@ulg.ac.be
- Bjorg Heringstad, Department of Animal and Aquacultural Sciences / Geno , Norwegian University of Life Sciences, Norway; bjorg.heringstad@umb.no
- Jennie Pryce, Agriculture Victoria and La Trobe University, Agribio Building, 5 Ring Road, Bundoora Victoria 3083, Australia; jennie.pryce@agriculture.vic.gov.au
- Kathrin F. Stock, IT Solutions for Animal Production (vit), Verden, Germany; Friederike.Katharina.Stock@vit.de
They were supported by the following claw health experts (in alphabetical order):
- Maher Alsaaod, University of Bern, Vetsuisse Faculty, Clinic for Ruminants, Switzerland; maher.alsaaod@vetsuisse.unibe.ch
- Nick Bell, University of London, Royal Veterinary College, Hatfield, Hertfordshire, United Kingdom; herdhealth@gmail.com
- Johann Burgstaller, University of Veterinary Medicine, Vienna, Austria, johann.Burgstaller@vetmeduni.ac.at
- Nynne Capion, University of Copenhagen, Copenhagen, Denmark; nyc@sund.ku.dk
- Anne-Marie Christen, Lactanet, Quebec, Canada; amchristen@lactanet.ca
- Gerald Cramer, University of Minnesota, College of Veterinary Medicine, St. Paul, Minnesota, USA; gcramer@umn.edu
- Gerben de Jong , CRV The Netherlands, Gerben.de.Jong@crv4all.com
- Dörte Döpfer, University of Wisconsin, School of Veterinary Medicine, Madison, USA; dopferd@vetmed.wisc.edu
- Andrea Fiedler, veterinary practitioner, Munich, Germany; dr.andrea.fiedler@t-online.de
- Terje Fjelddas, Norwegian University of Life Sciences, Norway; Terje.fjeldaas@nmbu.no
- Menno Holzhauer, GD Animal, Ruminants Health Department Health, Deventer, The Netherlands; m.holzhauer@gdvdieren.nl
- Johann Kofler, University of Veterinary Medicine, Vienna, Austria; johann.kofler@vetmeduni.ac.at
- Kerstin Müller, Freie Universität Berlin, Department of Veterinary Medicine, Clinic for Ruminants and Swine, Berlin, Germany; Kerstin-elisabeth.mueller@fu-berlin.de
- Hini Ruottu, Faba, Finland, hini.routtu@faba.fi
- Pia Nielsen, Seges, Denmark; pin@seges.dk
- Ase Margrethe Sogstad, TINE, Norway; ase-margrethe.sogstad@tine.no
- Gilles Thomas, Institut de l’Elevage, France; gilles.thomas@idele.fr
The working group acknowledges the valuable contributions and support of all the authors and contributors to the ICAR Claw Health Atlas (Egger-Danner et al. 2015[128]) and the review paper: 'Genetics and claw health: Opportunities to enhance claw health by genetic selection', published in the Journal of Dairy Science (Heringstad & Egger-Danner et al. 2018[129]).
Special thanks to Noureddine Charfeddine who led the development of these guidelines.
Annex 1: Risk factors for claw disorders
Claw disorders have a multifactor aetiology where risk factors for their occurrence could be deficiencies in housing systems and husbandry conditions, diet, hygiene, hoof trimming management, insufficient horn quality (for any reasons) as well as exposure to contagious agents and intoxications of certain minerals (Clarkson et al., 1996[130]; Bergsten, 2001[131]; van der Linde et al., 2010; Zinpro Corporation, 2014). A summary of the main risk factors related to the cow and related to the farm for infectious and non-infectious claw disorders are compiled in Table 24[1].
As for other health conditions, the most critical period regarding occurrence of claw disorders is the time around calving; therefore, besides general improvement of the cow’s environment, optimization of the transition period can be seen as an important factor for prevention.
A main farm risk factor for feet and legs problems is the type of surface the cows lay or walk on (Somers et al., 2003[132]). Most systems in Europe and North America have prolonged periods of time throughout the year where cattle are confined indoors, often on solid concrete or slats and fed conserved diets. If cattle do not have enough space for sleeping, walking and moving freely, longer periods of standing negatively impact claw health. Housing systems that do not allow appropriate consideration of the social status due to overstocking or too narrow walking paths or too few or uncomfortable cubicles increase the risk for claw disorders (Holzhauer et al., 2006[133]; Fiedler, 2015). Different roles of risk factors in pathways which lead to specific claw pathology may explain, why lower prevalence’s of foot lesions were reported for cows housed in tie stalls than for those housed in free stalls (Cramer et al., 2008[134]). Hygiene deficiencies on farm as well as contact between cows from different herds increase the risk for claw disorders related to infections like DD. Repeated contact to infectious agents may also contribute to the not consistently lower prevalence of claw disorders in cows with than without access to pasture: Regularly passed alleyways and too small pasture size bear the risk of cross-contamination, whereas claw health should generally benefit from opportunities of free movement on natural ground.
Some types of claw disorders are associated with diet composition. Rations with a high level of easily digestible carbohydrates and a high percentage of protein together with a low level of fibre may result in a disturbance of the digestion and increased risk of claw disorders.
The occurrence of claw disorders is also influenced by genetics, with some variation between the specific disorders. Therefore, in addition to improving management and nutrition, breeding for improved claw health is an important way of stabilizing and improving claw health. Breeding measures have the potential to achieve sustainable progress if enough emphasis is put on these traits in the breeding goal and the breeding program.
| Table 24. Risk factors and their associated claw disorders. | ||||
| Type of disorders | Risk factors | Preventive and risk effects | Associated disorders | |
| Infectious disorders | Cow-related factors | Calving
Age Breed Immunity system |
Around calving cows suffer stress and a depression of immunity system which favour the spread of infectious disorders. Young animals are most at risk as they have less developed immunity system.
Holstein-Friesian cows are more susceptible than other breed. The individual immunity response has been reported as a preventive factor against infectious disorders |
Digital dermatitis
Interdigital phlegmon Heel erosion Interdigital dermatitis |
| Farm-related factors | Cow comfort
Stall design Pen size Parlour capacity |
Cow comfort maximizes lying times and reduces stress. Reduces also contact with manure. Good stall design facilitates the cleaning process. | Digital dermatitis
Interdigital phlegmon Heel erosion Interdigital dermatitis | |
| Cow hygiene
Dry environment Slurry free environment |
Cleanliness reduces contact between pathogen and host.
Prevents introduction of infectious pathogens |
Digital dermatitis,
Heel erosion Interdigital dermatitis | ||
| Housing system
Access to pasture Straw yard |
Access to pasture or straw yard reduces infectious disorders and accelerate healing process | Digital dermatitis
Heel erosion Interdigital dermatitis | ||
| Diet | Diet affect immunity system mainly at early calving | Digital dermatitis
Interdigital phlegmon Heel erosion Interdigital dermatitis | ||
| Correct foot bath routine | Foot bathing aid in prevention of the initial infection and reduce the development of complicate infections | Digital dermatitis
Heel erosion Interdigital dermatitis | ||
| Non-Infectious disorders | Cow-related factors | Calving
Age Breed |
Disruptions to the growth of horn around the time of calving, which can lead to poor-quality horn formation | Sole hemorrhage
Concave dorsal wall Sole ulcer |
| Farm related factors | Cow comfort
Maximizing lying times Comfortable lying surface |
Reduces wear on the sole
Reduces pressure on the feet Reduces damage to the bony prominences |
Sole ulcer
Hock damage/swelling White line disease | |
| Housing system | Tied animals show less hoof lesions than those in loose housing. Free-stall barns mean long walking distances between the cubicles, feeding and drinking stations and the milking parlour. Good design and good walking surfaces might be the mitigate factors | White line disease | ||
| Flooring system
Walking and standing surfaces |
Rough and abrasive walking and standing surfaces lead to excessive wear and too smooth surfaces lead to slipping. Concrete floor has been shown to increase claw horn disorders. Rubberized walking surfaces in the feed alleys have been proven as preventive measures. | Sole ulcer
Heel ulcer Double sole Hock fissure White line disease | ||
| Social and physical integration for heifers and dry cows | Reduces defensive movements Avoids cow to cow confrontation. Reduces standing times
Improves eating and drinking behaviour |
White line disease | ||
| Cow flow on the farm
Good routes around Buildings To pasture To feed |
Allow a cow to express normal gait
Reduces defensive movements from humans to avoid confrontation Reduces standing times Improves eating and drinking behaviour |
White line disease
Sole ulcer | ||
| Diet
Macronutrients Micronutrients |
Not only the diet composition, but also the way it is prepared and fed. The reduction of ruminal acidosis and macro and micronutrient deficiencies or excesses improves hoof horn quality and integrity. | Sole haemorrhage
Concave dorsal wall White line disease Sole ulcer | ||
| Correct routine professional functional preventive hoof trimming | Corrects abnormal growth of the hoof horn
Prevents excessive/abnormal wear Prevents areas of deep sole horn Interrupts vicious circle of increased horn production Balances the weight load on lateral & medial claw Avoids high loading of localized areas of the sole |
Sole haemorrhage
Concave dorsal wall Hock fissure White line disease Sole ulcer | ||
Annex 2: Prevalence rates for claw disorders for different breeds in several countries
Table 25 shows prevalence rates for claw disorders calculated in different countries during 2015. In Finland, prevalence rates are calculated for Ayrshire and Holstein breed, while in The Netherlands parameters are calculated making distinction between first parity and multi-parity cows. Prevalence rates show a large variation between countries and illustrate some of the problems associated with between herd benchmarking. These differences could be explained by several reasons: Firstly, differences in the reporting level for some disorders, in fact within the same country the recording could be different across trimmers or practitioners. Secondly, the definition of claw disorders may not be completely the same. Thirdly, differences of the percentage of cows recruited for trimming. Finally, housing systems and weather conditions are different in these countries
| Table 25. Annual prevalence rates of claw disorders calculated in different countries and for different breeds and group of cows. | |||||||
| Denmark | Finland | France | Netherlands | Spain | Sweden | ||
| 1 | Interdigital Hyperplasia (IH) | 6.0 | AY: 1.5. HOL: 2.4 | 11.7 | COWS:6.0;HF:2.5 | 0.22 | 4.1 |
| 2 | Asymmetric Claws (AC) | 1.7 | AY: 0.1. HOL: 0.0 | 3.9 | |||
| 3 | Corkscrew Claws (CC) | 0.8 | AY: 8.6. HOL: 6.3 | 3 | 1.7 | ||
| 4 | Concave Dorsal Wall (CD) | 0,0 | 2.9 | 0.76 | |||
| 5 | Digital Dermatitis (DD) | 20.1 | AY: 0.8. HOL: 1.3 | 29.8 | COWS:21.0;HF:23.5 | 9.42 | 4.1 |
| 6 | Double Sole (DS) | 4.3 | AY: 1.4. HOL: 1.8 | 4.6 | 2.2 | ||
| 7 | Horn Fissure (HF) | 2.2 | |||||
| 8 | Vertical Horn Fissure (HFV) | ||||||
| 9 | Horizontal Horn Fissure (HFH) | ||||||
| 10 | Axial Vertical Fissure (HFA) | ||||||
| 11 | Heel Horn Erosion (HHE) | 10.8 | AY: 10.2. HOL: 11.4 | 54.5 | 17.2 | ||
| 12 | Interdigital Dermatitis (ID) | 2.3 | AY: 1.5. HOL: 2.5 | 1.41 | COWS:17.8;HF:10.6 | 6.9 | |
| 13 | Interdigital Phlegmon (IP) | 0.2 | AY: 0.4. HOL: 0.4 | 0.7 | 0.75 | 0.2 | |
| 14 | Scissors Claws (SC) | 0.7 | AY: 0.1. HOL 0.4 | ||||
| 15 | Sole Hemorrhage (SH) | 20.1 | AY: 16.4. HOL: 19.8 | COWS:24.2;HF:23.2 | 17.8 | ||
| 16 | Diffused Form (SHD) | 43.5 | |||||
| 17 | Circumscribed Form (SHC) | 16.2 | |||||
| 18 | Sole Ulcer (SU) | 6.1 | AY: 3.0. HOL: 5.7 | 5.8 | COWS:10.7;HF:4.0 | 12.87 | 4.8 |
| 19 | Typical Sole Ulcer (SUTY) | ||||||
| 20 | Bulb Ulcer (SUB) | ||||||
| 21 | Toe Ulcer (SUTO) | AY: 0.1. HOL: 0.2 | 0.1 | ||||
| 22 | Toe Necrosis (TN) | 0.7 | 1.8 | ||||
| 23 | Swelling of the Coronet and/or the Bulb (SW) | ||||||
| 24 | Thin Sole (TS) | ||||||
| 25 | White Line Disease (WLD) | 15.1 | COWS:21.0;HF:12.9 | 8.85 | |||
| 26 | WL Fissure (WLF) | 8.2 | AY: 10.1. HOL: 13.1 | 2.2 | |||
| 27 | WL Abscess/Ulcer (WLA) | 2.5 | AY: 1.0. HOL: 1.5 | 0.4 | |||
| All lesions | COWS:61.9; HF:43.4 | 30.51 | |||||
[1] Mülling et al. 2006[135]; Palmer et al. 2015; Barker et al. 2009.
Lameness in Dairy Cattle
About this Guideline
The Guidelines for recording lameness in dairy cattle give an overview of the most common systems of lameness scoring and recording in dairy cows. They are important components of lameness control strategies on dairy farms. Lameness scoring, when applied on a regular basis, allows detection and treatment of lame individuals at an early stage of disease. Collected data can be used to evaluate the herd’s lameness control strategy and provide information for further analyses and research. The guidelines include considerations and recommendations for improved lameness recording in the context of a herd health management program, animal welfare, benchmarking and genetic evaluation.
Terminology
Lameness scoring will be used in this document. Other terms such as locomotion scoring, mobility scoring, and gait behaviour or gait assessment are used for similar traits. These are distinct from locomotion scoring as referred to Section 05 of the ICAR Guidelines for conformation recording.
Recommendations of Lameness Recording Practices
SYSTEM: A five-scale system (1 to 5) which considers different aspects of posture and gait (arched back, head bob and signs of weight bearing on non-affected limbs) – Table 26.
USERS: Dairy farmers, veterinarians, hoof trimmers, dairy advisors and farm employees.
HOW MANY: If cows are housed in pens, the number of animals selected for assessment should be proportional to the number of cows in each pen. A strategic sampling would be to assess cows from the middle of the milking order; the number being associated to the size of the herd. On large pasture-based herds, it is recommended that the last 200 cows should be assessed as a screening test.
HOW: Score lameness on a flat, firm, and non-slippery surface on which the cows are expected to walk normally or familiar to. While cows are walking, the assessor should view the animals from the side. Cows must not be assessed when they are turning. Animals to be assessed should be randomly chosen.
WHEN: Assessing cows after milking is the best time for scoring lameness. The environmental conditions should be as calm as possible to allow cows to walk as they would normally.
HOW OFTEN: For herd management:
- Optimally, every two weeks, at least once a month;
- For early detection of hoof health problems: weekly or every two weeks is recommended;
- If monthly assessment is not feasible and if no routine claw trimming is taking place: at dry-off and at the beginning of lactation.
For genetic evaluation:
- If possible, use of data collected for herd management (single or multiple records per cow and lactation).
KNOW-HOW: Short theoretical instructions on the description of the five lameness categories and practical basic training is needed. Annual training of assessors is highly recommended.
4:Ref.: Sprecher et al. 1997 [136]/ Source of the pictures: Zinpro First Step®: Dairy Lameness Assessment and Prevention Program.
Introduction
Locomotor diseases causing lameness are widely recognised as one of the most serious welfare issues for dairy cattle and they represent substantial costs for dairy farmers (von Keyserlingk et al., 2009[137]). Lameness indicates pain or discomfort during locomotion and is characterized by a change in gait or an irregularity of the walking pattern. Lameness is most often caused by claw and/or leg disorders reflecting the attempt of the animal to reduce the amount of weight bearing on the affected limb(s). Therefore, lameness is considered as an indicator of an underlying problem that often causes pain (Flower & Weary, 2009[138]). Lameness is associated to lower dry matter intake, impaired milk production and reproduction, and can lead to early culling. Thus, by reducing a cow’s mobility, overall health and welfare are impacted.
The majority of lameness cases in dairy cattle are related to lesions of the claws, infectious or non-infectious (Toussaint Raven, 1978), that induce pain. According to Green et al. (2002)[139], 80-90% of causes of lameness in cattle are located in the distal limb. Claw diseases occur most frequently in the first 3-5 months post-partum. In North American dairy herds, the main causes of lameness are sole ulcers, white line disease, toe ulcers, digital dermatitis, foot rot, and thin soles (Bicalho et al., 2007[140]; Sanders et al., 2009[141]; DeFrain et al., 2013[142]).
In a field study done in 2013 and 2014 by University of Calgary, Canada, veterinarians looked at the relationship between claw lesions and lameness in 10 dairy farms (Douglas et al., 2019[143]). Results showed that on average, 20% of cows were lame. A lesion was present in 94% of all lame cows and in 84% of non-lame cows. A cow with a lesion was almost three times more likely to be lame than a cow without a lesion. Results suggest that a cow with a sole ulcer or a white-line lesion was 12 to 13 times more likely to be identified as lame, whereas a cow with digital dermatitis (DD) was three times more likely to be identified as lame. The fact that six to eight weeks pass before damage of the corium becomes visible at the sole horn explains the low correlation between lesion presence and lameness detection. In this study, 84% of non-lame cows showed a lesion, putting them at higher risk for becoming lame.
The type of lesion influences lameness prevalence differently; cows with a sole ulcer or white-line lesion having a greater chance of being identified as lame than those with DD. Then, recording claw lesions during trimming would be an optimal practice for monitoring and preventing more serious claw diseases or limb disorders.
Consequently, prevention methods such as frequent lameness scoring are effective for:
- Early detection of claw lesions and feet and leg disorders;
- Monitoring lameness prevalence;
- Comparing lameness incidence and severity between herds;
- Targeting individual cows that need hoof trimming.
Other potential underlying conditions causing lameness include joint disorders (e.g. arthritis, arthrosis, luxation), diseases of muscles and tendons (e.g. myositis, tendinitis), and neurological diseases (e.g. neuritis, paralysis). Genetics can play a role for occurrence of lameness through disposition to aforementioned disorders or malformations such as corkscrew claws or similar deformations.
The environment of the cows can increase the risk of lameness such as housing, including type of flooring, and herd management practices (Solano et al., 2015[144]). In Australia, New Zealand and South America where the dairy industry is predominantly pasture-based, cows may often walk several kilometres and stand for several hours per day in a crowded concrete yard while they wait to be milked. The potential for lameness to negatively affect animal welfare is of ongoing concern (Beggs et al., 2019[145]; Hund et al, 2019[146]). Pressure applied when walking down to dairy and when in the yard from excessive/incorrect use of backing gate may induce lameness. Cows should be left to walk to and away from the dairy at their own pace and the backing gate should be used only to fill space in the yard - not to push cows up.
The risks factors most commonly associated with lameness are:
- Walking and standing on concrete, especially wet and rough;
- Walking long distance on poor walking surfaces;
- Lack or absence of appropriate bedding and bad hygiene;
- Poorly designed stalls;
- Overcrowded pens;
- Pressure applied when walking to and away from the dairy and incorrect use of backing gate;
- Overcrowded pens and poor cow traffic;
- Infrequent and/or incorrect claw trimming;
- Insufficient monitoring that results in late detection of cows requiring additional care;
- Poor management, particularly of transition cows;
- Insufficient body condition (<2; Randall et al., 2015 [147]/ For reference, see the Section 5 of the ICAR Guidelines for conformation recording);
- Parity;
- Physical hazards.
Preventing lameness helps to optimize milk production, improves conception rates and animal welfare and reduces treatment costs and antibiotic use. Consequently, it lowers stress level in both, cows and dairy farmers. However, improving gait/locomotion requires detailed information on individual lameness cases and informative records helping to identify causative factors that need to be eliminated or corrected.
The use of detailed information from veterinarians (for more severe lameness cases) and hoof trimmers (screening data and less severe cases) may allow deeper insight into improvement options. As various disorders are demonstrated to be related to certain risk factors, recordings obtained at routine claw trimming and treatment of lame cows allows for targeting on-farm risk assessment enabling farmers to alleviate or even eliminate potential risk factors.
Lameness Scoring Methods
Subjective methods are currently used for assessing cows on farms, and the results are described as numerical rating scores. It rates individual cows for the presence or absence of certain behaviours and postures related to gait. These scoring systems focus mainly on locomotion or gait associated with the degree of reluctance of bearing weight on the affected limb(s) with five, four or even only two categories (Brenninkmeyer et al., 2007[148]).
Over time, results from different studies show that subjective scoring can be applied consistently within and among observers, especially if the scoring system provides a detailed definition of each category and if the observers/assessors have been trained (Flower & Weary, 2009[149]). Despite lack of precision, simple recording of lame animals by dairy farmers, advisors or veterinarians may be the easiest system for recording lameness on a routine basis. However, it is most reliable for cows that are either moderately lame, lame or severely lame (Sogstad et al., 2012[150]). Lameness scoring should be seen as a complement to the recording of claw health information during routine claw trimming for early detection of individual cows with problems in between trimmings.
Recording lameness may be performed on different levels of specificity and for different purposes. According to the objectives, some systems refer as being either a lameness scoring system or a mobility scoring system. A specific system is used for scoring lameness in tie-stall barns.
The Sprecher system: Scale of 1 to 5
The most popular systems for scoring lameness rely on the Sprecher system. This is a five-point scale system widely recognised and used worldwide due to its simplicity and the observation of the presence of behaviours such as an arched back when standing and walking (Sprecher et al., 1997[151]). This scoring system, where 1 is «normal» and 5 is «severely lame», is non-invasive and easily applied under farm conditions with short theoretical instructions and subsequent practical training. It allows more individuals to perform this assessment such as dairy farmers and their employees, veterinarians, hoof trimmers and advisors. Then, this scoring information can be used for herd management and early detection of lameness.
A similar approach uses behavioural variables or production variables as indicators for impaired gait (Schlageter-Tello et al., 2014[152]). The «Zinpro First Step®: Dairy Lameness Assessment and Prevention Program» uses that 1 to 5 scale to assess the severity of dairy cattle lameness. It is based on the observation of cows standing and walking (gait), with a special emphasis on their back posture. A combination of the Sprecher system and the «Zinpro First Step®» is presented in Table 1 and is the reference standard proposed for the current Guidelines.
However, in large herds such in Australia and New Zealand, a similar system is used where 0 means «Walks evenly» and 3, «Very lame». This system called «mobility scoring system» is also used in the UK and the US and is summarized at APPENDIX 1. A correspondence can be made between the mobility scoring system and the one presented on Table 26 where:
| Mobility Scoring System | Table 26 |
| Score 0: Walks evenly | Score 1: Normal |
| Score 1: Walks unevenly | Score 2: Mildly lame |
| Score 2: Lame | Score 3: Moderately lame |
| Score 3: Very lame | Score 5: Severely Lame |
There are other scoring or assessment systems used in different countries and for different purposes and they are described in 5.11 (Appendix 1):
- «Welfare Quality Network» with a scale of 0 to 2;
- «Gait behaviours for non-lame and lame cows»;
- «König-Garcia mobility score»;
- «Stall lameness score system (SLS): 0 for non-lame cow and 2 for lame cows.
Some considerations for recording lameness
Training of the observers
Training is the main factor assuring proper performance of the observers at lameness scoring. Improved agreement across observers is obtained as more cows are assessed (March et al., 2007[153]). In this study, the authors suggested that 200 to 300 cows are sufficient numbers to score for reaching the acceptance threshold for agreement and reliability when using a five-scale system. Even after obtaining the acceptance threshold, observers should receive periodic training to avoid any “drift” which refers to the tendency of observers to change over time how they apply the definition of a measurement. A periodic training would be defined by once or twice a year alternating between practical exercise and online training for example.
Generally, training is crucial for achieving high agreement levels. It should be designed depending on the level of precision that is required. For example, the integration of a 5-scale gait scoring system into on-farm welfare assessment protocols is seen as justified, if adequate practical learning phase is assured (March et al., 2007[153]). However, Garcia et al. (2015)[154] demonstrated that contrary to the current belief, the highest level of experience was not necessarily associated with a higher chance of perfect agreement.
How many animals should be assessed?
It is important to recognise that the ideal approach to assess the levels of lameness within a milking herd is to assess all cows. This approach highlights the potential animal welfare benefits of formal and systematic lameness scoring of dairy herds for improving identification and treatment of lame cows (Main et al. 2010; Beggs et al. 2019[155]).
Studies have shown that random sampling during milking conveys limited practical benefits and oblige the assessor to be present throughout the milking (Main et al. 2010). Farm size may be a barrier to farmers participating in lameness scoring of the whole herd. A simpler alternative sampling strategy would be an incentive to do it more frequently.
Main et al. (2010) suggested a sampling based on getting within 5% of the true prevalence (Table 27). This study suggested that sampling herds from the middle of the milking order on most farms would seem most appropriate.
| Table 27. Sampling based on the quadratic equation that best explained the sample size needed to get within 5% of the true prevalence based on sampling cows from the middle of the milking order. | |
| Herd size | Sample size* |
| 25 | 20 |
| 50 | 30 |
| 75 | 40 |
| 100 | 49 |
| 125 | 57 |
| 150 | 64 |
| 200 | 75 |
| 225 | 79 |
| 250 | 82 |
| 275 | 84 |
| 300 | 85 |
* Sample size = −0.001n2 + 0.498n + 6.785, where n = number of cows in milking herd.
In large pasture-based herds, Beggs et al. (2019)[156] indicate that lameness scoring at least 200 cows at the end of the milking order would give some confidence that the overall lameness prevalence is correct. This number is useful as a screening test, identifying herds that were likely to have lameness prevalence above a given threshold. Presence of severely lame cows at the end of milking order may also be useful for identifying those farms likely to benefit from further support. But on a practical point of view, this recommendation would require dedicating resources on that specific task. Farmers are taught to look for lame cows every time they come into milking, at milking and when walking out.
Walking surface and location
Several studies indicate that the surface conditions in the walking area (soil and flooring) can have profound effects on gait. In a study, gait of cows walking on sand was compared to gait on slatted and solid concrete flooring. On slatted concrete floor, cows walked more slowly with considerably shortened strides and with the rear feet placed at greater distance behind the front ones. On the solid concrete floor, cows took shorter strides and steps than on the sand surface, but the speed did not differ significantly. Rubber mats on concrete floor increased the length of strides and steps and had a positive effect on locomotion in both, lame and non-lame cows (Telezhenko & Bergsten, 2005[157]).
Concrete is not an ideal surface for dairy cows to walk on despite it being the most common surface found on farms. It could lack sufficient grip for cows to move around comfortably without fear of slipping. Grooving is therefore essential for a good traction, but a compromise has to be struck between sufficient grooves for allowing traction and too many grooves that would cause excessive wear (Cook, 2005[158]).
Rubber flooring provides a more secure footing and is softer and more comfortable to walk on, especially for lame cattle (Flower et al., 2007[159]).
Consequently, lameness scoring should be performed with cows walking on a flat, firm, and non-slippery surface. To gain consistency and reliability of scores on subsequent visits on the same farm ideally the same way, the same location and same walking surface should be used for scoring. For example, when the parlour exiting routine becomes disrupted, cows will often not show their normal behaviour and are more likely to conceal lameness (Groenevelt et al., 2014[160]).
How often and when
To correctly identify new cases of lameness and for early detection of claw health problems, it is preferable if monitoring of lameness is performed every two weeks (Eriksson et al. 2020 [161]– In press). Several studies concluded that lameness and locomotion scores may be useful indicator traits for claw health (Laursen et al., 2009[162]; Weber et al., 2013[163]; Egger-Danner et al., 2017[164]). Decreased assessment frequency can make it more difficult to adequately identify new lame animals (Eriksson et al. 2020 [165]– In press). In addition to lameness assessment every two weeks, immediate treatment of lame cows will lead to reduced lameness prevalence. Early treatment of lame dairy cows results in the development of less severe claw lesions, increasing the chance of full recovery and decreased the amount of time an animal was lame (Groenevelt et al., 2014[160]).
In the near future, new technical advances (e.g. sensors. pedometers or accelerometers) could make it possible to monitor the gait of dairy cows in real time such that lame cows could be treated immediately (Haladjian et al., 2018[166]). Examples of behaviours that may be associated with lameness include walking speed, lying time, etc.
It is especially important to assess lameness at dry off and at the beginning of lactation if no routine claw trimming is taking place in the herd. If there are lesions, it is important that these can heal during the dry period such that the animal does not enter a new lactation with existing foot health problems. As not all claw disorders are correlated to lameness, claw trimming is recommended when cows enter the dry period and at approximately two months post-partum (Kofler, 2015[167]). In a study, Ahlén & Fjeldaas (2019)[168] showed that locomotion scoring was insufficient to detect and control digital dermatitis in Norwegian free stall herds and that inspection in trimming chutes was necessary to detect the disease.
The most suitable time to assess lameness is right after milking because it is more compatible with normal farm work routines. The assessment should not disrupt cows outflow routine to be sure they keep a normal behaviour. To support that practice, results reported by Flower & Weary (2006)[169] showed that for cows with and without sole ulcer, the differences in gait before and after milking were evident. After milking, all cows had a significant improved gait. This change was probably due to udder distention and/or motivation to return to the home pen.
Finally, the use of detailed information from veterinarians (for more severe cases) and hoof trimmers (screening data and less severe cases) may allow deeper insight into improvement options. As various disorders seem to be related to certain risk factors, information obtained during routine claw trimming and treatment of lame cows allow for targeting on-farm risk assessment in order to alleviate or even eliminate potential risk factors.
How to Score Lameness
Including lameness scoring in routine herd management is the most practical way for detecting lameness in dairy cattle on farms. This method or practice can be used in free-stall or other types of loose-housing systems and in tie-stall systems where cattle are routinely exercised, if practical. The lameness scores are ideally entered into a herd management software or can be recorded using a board and a paper recording sheet. Appendix 2 presents two examples of data recording sheets.
Instructions for a free-stall barn
Identify a suitable location
Often the easiest location on the farm is the passage between the milking parlour and the pens. The criteria for choosing an adequate location are:
- Distance allows observation of cattle walking for four strides (minimum of two strides);
- Surface is smooth/flat and allows long confident strides without slippage;
- Avoid slatted concrete surfaces if possible;
- Avoid sloped flooring (downward or upward) or alleys with steps.
If cattle have been released from tie-stalls for allowing the scoring, habituate them to walking by walking up and down a passageway in a calm manner until the cattle walk in a straight line at a steady pace.
Identification of the animal
Record the identification of the cow to be assessed in the data-recording sheet:
- Ear tag number;
- Neck number.
Lameness score the cow
Observe at least four strides for each animal and record the degree of limping/reluctance of bearing weight on the affected limb(s) of the cow. Score and record information on the data-scoring sheet. Appendix 2 presents examples of recording sheets.
Instructions for a tie-stall barn
- Assess standing cows
- Encourage all cows to be assessed to stand for at least 3 minutes before their assessment begins. Do not score if the cow urinates or defecates during the assessment.
- Identification of the animal
- Record the identification of the cow to be assessed in the data-recording sheet.
- Observe
- Observe the cow for lameness. The assessment consists of two parts:
| A. Assessment of foot placement – Standing Pose | |
| 1. Observe the foot position and placement of the cow for a full 10 seconds in each of the following three positions: | |
| • Directly behind the cow such that both legs are visible (about 0,5-1m behind the stall) | |
| • Left of the cow for a side-view of both legs | |
| • Right of the cow. | |
| 2. Record the presence of EDGE, SHIFT and REST indicators for each position (Ref.: Table 29). | |
| B. Shifting of the cow from side to side | |
| 1. Position yourself behind the cow with a view of both front and hind feet. | |
| 2. Ask the producer to shift the cows from side to side: | |
| a. | • First walk from the right to the left behind the cow and then back to the right |
| b. | • If the cow does not respond to your movement, repeat this while tapping her hip bone, with your hand, on the side opposite to where you want her to move (i.e. If you want her to move left, tap her right hip bone) |
| c. | • If this still does not work, poking gently with the tip of a pen may replace a tap. |
| 3. Pay attention to how the cow shifts weight from foot to foot | |
| d. | • Observe if the UNEVEN indicator is present. This can be identified as a reluctance to bear weight on a particular foot*[1] |
| e. | • Observe the foot position and placement and the presence of EDGE, SHIFT and REST indicators resumed after movement. |
| 4. Record presence of behavioural indicators in the Data Recording Sheets. | |
Score cows
A cow will be scored as obviously/severely lame (unacceptable) if 2 or more indicators are recorded. Record either «Lame» or «Not lame» on the recording data-sheet.
Use of Lameness Data
A precondition for use of lameness records for benchmarking, herd management and genetic evaluation is the storage of the information collected on farms into a central data base.
Herd Management
Lameness records are valuable information for early detection of claw problems. Claw trimming data are essential for the identification of the specific problem(s) and for targeting corrective measures (Fjeldaas et al., 2011[170]; Kofler, 2013[171]). According to Green et al. (2002)[172], lameness prevalence is highest in early lactation cows. In Austria, a study related to the «Efficient Cow Project» (Egger-Danner et al., 2017[173]) involving about 7,000 cows with lameness records assessed according to the Sprecher system at each milk recording test across a lactation, revealed rather stable incidences across the lactation.
According to Randall et al. (2018)[174], between 79 and 83% of lameness events were estimated to be attributable to all previous lameness events and between 9 and 21% attributable to exposure to lameness events that occurred at least 16 weeks previously. Then, preventing the first case of lameness could potentially be important in avoiding an escalation of repeated lameness events. In addition, findings from this study highlight that early and effective treatment of lameness reducing the likelihood of recurrence or cases becoming chronic may also be crucial to lameness control at a herd level.
Benchmarking
A precondition for the use of lameness records for benchmarking, herd management and genetic evaluation is the storage of the information collected on farms into a central data base.
Benchmarking is important for herd management as it ranks the farm amongst its peers and it helps identifying where improvement is needed. However, to be able to compare herds, the frequency of assessment, the stage of lactation and the recording scheme itself need to be considered. Animals at risk need to be defined based on the strategy of data recording. If assessment of lameness is done every month or even more often, the frequency will most likely be higher compared to an assessment that is done once in lactation, or once a year at herd level. Therefore, the interpretation of results needs to take into account the circumstances of recording. The reference population will need to be defined and the criteria for claw health considered.
Welfare
It is well recognised that lameness is a painful experience for the cow (Whay et al., 1997[175]), causing loss of milk yield, poor fertility and body condition. The presence of lame and ill cattle in the milk-producing herd erodes consumer confidence in dairy farmers and farming practices. Despite increased awareness of lameness in relation to welfare and lost productivity, no studies reported a reduction in the prevalence of lameness over the last 20 years (Heringstad & Egger-Danner et al., 2018[176]). There are a number of barriers to improvement in the prevalence of lameness. Firstly, dairy farmers must recognise lameness. Studies have shown that without training, farmers will detect mainly the severely lame cows (Whay et al., 2003[177]; Leach et al., 2010[178]). Secondly, dairy farmers must find the time to observe the locomotion of all their cattle at frequent intervals. For them, shortage of time is a major obstacle to the use of visual lameness scoring as a tool for reducing lameness (Leach et al., 2012[179]). However, providing dairy farmers with training to detect all states of lameness, and the use of incentives for reducing lameness would improve the situation.
To encourage dairy farmers to carry out lameness assessments, a number of organisations included lameness assessments within a welfare assessment scheme. Among those organisations are increasing numbers of retailers, milk processors and other food groups that now include aspects of animal welfare in their assessment schemes. The schemes are designed to provide assurance to the consumers about the standards of animal welfare. Lameness is one of the most commonly used welfare indicators in these schemes. Recording lameness as an indicator of welfare is a very valuable method to raise awareness and its negative impact for the dairy farmers and the public. However, there is a variation between schemes in the scale used for scoring animals, some only score a limited proportion of the herd and some do not record the identity of the animal, which are aspects that require improvement for allowing wider use of the data.
Genetics
Lameness records are valuable auxiliary traits for genetic improvement and should, if possible, be combined with claw trimming records, veterinary diagnoses and other existing information (e.g., culling for claw health, linear scoring) as lameness information itself does not give an indication of the causative disorder. Ring et al. (2018)[180] and Egger-Danner et al. (2017)[181] showed positive genetic correlations between lameness and direct claw health traits.
Animals at risk need to be identified and checked whether there is variation in the type of scoring scale used. The frequency of scoring has to be considered for the choice of the model. If repeated lameness scores are available per cow and lactations, trait definitions and models need to be optimised.
Trait definitions depend on the scale used. Several studies (Berry et al., 2010[182]; Parker Gaddis et al., 2014[183]; Koeck et al., 2016[184]) used lameness observations, coded «0» (not lame) or «1» (lame), in a comparable manner to certain health disorders recorded by farmers. In other cases, lameness can be grouped into three different scores (non-lame, lame and severely lame cows). Definitions might take into account the frequency of the occurrence of different scores as well as the frequency of recording (Koeck et al., 2018[185]). If the lameness data recorded will be used for herd management purposes, then data quality has to be especially verified (see this section, Section 7 of the ICAR guidelines).
An important question is the definition of the contemporary group:
- Is lameness recorded from all animals or only for the lame cows?
- Is the trait definition across farms comparable?
- Are the same standards used?
The severity of lameness may also be described using a clinical gait score (Sprecher et al., 1997[186]; Flower & Weary, 2006[187]; Koeck et al., 2016[188]; Egger-Danner et al., 2017[189]), which quantifies lameness on a scale from absent to very severe. For analysis, the severely lame cows (scored 3 or higher) may be analysed jointly (e.g. Rouha-Muelleder et al., 2009[190]; Weber et al., 2013[191]).
In a review, Heringstad & Egger-Danner et al., (2018)[192] reported heritability estimates of lameness varying between 0.02 and 0.16 based on linear models and from 0.02 to 0.15 based on threshold models. Berry et al. (2011)[193] reports heritabilities for lameness varying from 0.03 to 0.096 when scored by farmers or by trained assessors. The genetic correlations between lameness and claw health were between 0.60 and 0.95 (Heringstad & Egger-Danner et al., 2018[194]; Ring et al., 2018[195]). Most genetic correlations between production and lameness are unfavourable. The relationship of lameness and claw health with milk production is complex as it is difficult to distinguish causes from effects (Heringstad & Egger-Danner et al., 2018[196]).
Koeck et al. (2019)[197] showed that selecting for a better lameness score has the potential to reduce claw diseases, especially the frequency of severe claw diseases that lead to culling. As recording systems include lameness data as integral parts of routine welfare assessments on farms, and more and more farmers use lameness scoring for herd management purposes, increased availability of data may be expected in the future.
[1] Cows with sole ulcers or white line lesions on the lateral hind claw often try to relieve pain by putting more weight on the medial claw.
Contributors
ICAR gratefully acknowledges the contributions to this lameness guideline by the following people:
| • Anne-Marie Christen, Lactanet, Canada |
| • Christa Egger-Danner, ZuchtData, Austria |
| • Nynne Capion, University of Copenhagen, Denmark |
| • Noureddine Charfeddine, CONAFE, Spain |
| • John Cole, USDA, United-States |
| • Gerard Cramer, University of Minnesota, United-States |
| • Gerben de Jong, CRV Holding, Netherlands |
| • Andrea Fiedler, Hoof Health Practice, Germany |
| • Terje Fjeldaas, Norwegian University of Life Sciences, NMBU, Norway |
| • Nicolas Gengler, Gembloux Agro-Bio Tech, Université de Liège, Belgium |
| • Marie Haskell, Scotland Rural College, Scotland |
| • Bjørg Heringstad, Norwegian University of Life Sciences, NMBU, Norway |
| • Menno Holzhauer, GD Animal Health, Netherlands |
| • Astrid Koeck, ZuchtData, Austria |
| • Johann Kofler, University of Veterinary Medicine, Austria |
| • Kerstin Müller, Freie Universität, Germany |
| • Jenny Pryce, La Trobe University, Australia |
| • Åse Margrethe Sogstad, TINE, Norway |
| • Friederike Katharina Stock, Vereinigte Informationssysteme Tierhaltung w.V. (vit), Germany |
| • Gilles Thomas, Institut de l’Élevage, France |
| • Elsa Vasseur, Mc Gill University, Canada |
Appendix 1: Alternative Scoring Systems for Lameness
Mobility scoring system: Scale of 0 to 3
A mobility scoring system is used in the UK (AHDB Dairy), in New Zealand (DairyNZ) and in Australia (Dairy Australia) where herds are large and cows are grazing most of the year. It is also promoted in the FARM Program in the US. It was designed so that anyone with experience of working with dairy cattle is able to perform mobility scoring effectively. The mobility scoring system is a four-point scale ranging from 0 «Walks evenly» to 3 «Severely or very lame». It simply assesses the cow's ability to move easily. By simplifying the scoring system, the aim is that dairy farmers are able to easily assess cow mobility on farm without the need for professional help.
The Welfare Quality Network: Scale of 0 to 2
This European organisation focuses on scientific exchange and activities to contribute to the development of the Welfare Quality® animal welfare assessment systems. A Welfare Quality® assessment protocol for cattle was developed for scoring lameness and proposes a 3-point scale program where 0 is «Not lame» and 2 is «severely lame». No specific target is proposed for each point.
Gait behaviours for non-lame and lame cows
Table 28 presents the general description for a two-scale program for scoring lameness: Lame or non-lame. This program is based only on gait behaviours and assessors must rely on evident signs of body language for determining the status of lameness of animals.
| Table 28. General description of gait behaviours for non-lame and lame cows. | ||
| Behaviours | Non-Lame Cows | Lame Cows |
| Head bob | Up and down head movement when walking. The head moves evenly as an animal walks. | Jerky or exaggerated up and down head movements when walking. Obvious when foot makes contact with ground |
| Asymmetric steps | Animal places her feet in an even “1, 2, 3, 4” fashion | Animal has uneven rhythm of foot placement “1, 2…..3, 4”. Foot placement is not equal on both sides |
| Limping | Animal bears weight evenly over the four limbs | Walk with an uneven, irregular, jerky or awkward step as if favoring one leg |
www.dairyresearch.ca/pdf/3-Animal%20Based%20Protocols-Dairy%20Research%20Cluster-eng.pdf
König-Garcia mobility score
König-Garcia et al (2015)[198] developed a five-scale scoring system named: the König-Garcia mobility score. This system was specifically developed to enable scoring while walking only because it is difficult to get an opportunity to see cows standing and walking under practical conditions. This mobility scoring achieves relatively high within-observer agreement and seems feasible for on-farm implementation as a tool for monitoring mobility for benchmarking of lameness prevalence.
Stall lameness score system (SLS): 0 for non-lame cow and 2 for lame cows
In tie-stall barns, scoring lameness can be challenging because cows may not be used to walking and there may not be a suitable area in which to walk cows. If walking and observation of cows is not possible, a stall lameness score system should be used.
This system represents an easier approach for scoring dry cows and young stock. SLS can be conducted in automated milking systems when cows are fixed during milking time to detect lame or affected cows. The SLS is based on a number of behaviours that cow shows while standing in the tie-stall (Winckler and Willen, 2001[199]; Leach et al., 2009[200]; Gibbons et al., 2014 [201]- Table 29).
The most common behaviours recorded are:
- Weight shifting;
- Standing on the edge of the stall;
- Uneven weight bearing while standing, and;
- Uneven weight bearing while moving from side to side.
The SLS method provides an estimate of the prevalence of lameness in tie-stall herds comparable with traditional gait scoring, but does not require that the cows be untied. It could be used to improve lameness detection on tie-stall farms and obtain estimates of lameness prevalence without the need to walk the cows (Gibbons et al., 2014[201]).
| Table 29. Description of the behaviour indicators of the stall lameness score system[1]. | |
| Behaviour indicator | Description |
| Standing Pose (Voluntary movements) | |
| Stand on Edge
(EDGE) |
Placement of one or more feet on the edge of the stall while standing stationary.
Standing on the edge of a step when stationary, typically to relieve pressure on one part of the claw. This does not refer to when both hind feet are in the gutter or when cow briefly places her foot on the edge during a movement/step. |
| Weight shift
(SHIFT) |
Regular, repeated shifting of weight from one foot to another. Repeated shifting is defined as lifting each hind foot at least twice off the ground (L-R-L-R or vice versa).
The foot must be lifted and returned to the same location and does not include stepping forward or backward. |
| Uneven weight
(REST) |
Repeated resting of one foot more than the other as indicated by the cow raising a part or the entire foot off the ground. This does NOT include raising of the foot to lick or during kicking. |
| Cow moved from side to side | |
| Uneven movement | Uneven weight bearing between feet when the cow was encouraged to move from side to side. This is demonstrated by a greater rapid movement of one foot relative to the other, or by an evident reluctance to bear weight on a particular foot. |
Future Measures of Lameness
Development of gait assessment or automatic lameness detection systems could provide more accurate and reliable data in the near future. Currently, these technologies are mostly used in research and they require sophisticated equipment or installation that limits their large-scale use on farms. Some examples of such technologies include 3D images-based systems, thermal imaging cameras, 4-scale weighing platform, or wearable activity sensors (Alsaaod et al. 2015[202]; Beer et al. 2016[203]; Nechanitzky et al. 2016[204], Barker et al. 2018[205]).
Using an activity sensor to measure, inter alia, lying time, tools for automatic lameness detection can estimate the risk of lameness by employing special models that take milking and feeding times into account (De Mol et al. 2013[206]). Beer et al. (2016)[207] reported that compared to healthy, non-lame cows, the behaviour of lame cows or cows with foot pathologies was characterized by longer lying bouts, more time spent lying down, shorter strides, slower walking speed, lower bite rate while grazing, and lower feeding time or faster eating. Models based on only two 3D accelerometer variables (walking speed, standing bouts) automatically identified slightly lame cows with both a sensitivity and specificity exceeding 90% (Beer et al. 2016[207]).
Giuliana et al. (2014)[208] showed that lameness leads to behavioural changes in automatic milking systems. A recent study showed that a 4-scale weighing platform allowed the detection of cows with sole ulcers or white line disease with a sensitivity of 97% and a specificity of 80% (Nechanitzky et al 2016[204]). Recently, infrared thermography (IRT) has been used in bovine medicine to identify thermal skin abnormalities by characterizing a temperature increase or decrease in affected areas. The variation in superficial thermal patterns resulting from changes in blood flow, in particular, can be used to detect inflammation or injury associated with conditions such as foot lesions (Alsaaod and Büscher 2012[209]; Stokes et al. 2012[210]; Alsaaod et al. 2014[211]; Wilhelm et al. 2015[212]).
These technologies are still costly and still under development for increasing accuracy and precision for detecting abnormalities in cow gait or posture.
Appendix 2: Data Recording Sheets for lameness
Data Recording Sheets
A greater understanding of the dynamics of lameness in dairy herds can be obtained from improved record keeping systems and a comprehension of how lame cows interact with the environment (Cook, 2005[213]). The dairy farmers or herd manager needs to determine the extent of the lameness problem on his herd:
The predominant causes;
Their trigger factors, the risk factors, and,
To understand the role of cow comfort and adequate hoof care.
Figure 19[2] and Figure 20 present proposed templates for recording lameness in free- and tie-stall barns respectively.
| Figure 19. Example of a data-recording sheet – Free-stall. | ||||||
| Cow ID | 1 Normal | 2 Mildly lame | 3 Moderately lame | 4 Lame | 5 Severely lame | |
| 1 | ||||||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | ||||||
| … | ||||||
Note: 90% cows = score 1 / <10% cows = scores 2 + 3
| Figure 20. Example of a data-recording sheet – Tie-stall. | ||||||
| Cow ID | Stand on edge | Weight shift | Uneven weight | Uneven movement | Severely lame | |
| 1 | ||||||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | ||||||
| … | ||||||
Note: A cow will be scored as obviously/severely lame (unacceptable) if 2 or more indicators are recorded.
[1] Ref.: Gibbons, et al. 2014.
[2] Both adapted from the Dairy Research Cluster (www.dairyresearch.ca/cow-comfort.php#self).
Calving traits in Dairy Cattle
Introduction
The purpose of these ICAR guidelines for recording of calving performance traits in dairy cattle is to give recommendations on recording, data validation and use of information in herd management, documentation of animal welfare, benchmarking, and genetic evaluations. For beef breeds please see Section 3 of the ICAR guidelines for Beef Cattle Recording.
Definitions and terminology
The main calving traits are stillbirth and calving ease. Other relevant traits are calf size and gestation length. All these traits have both direct and maternal aspects.
Stillbirth is one of the major issues related to the calving. Figures suggested that the frequency has increased in dairy herds, although the reasons are still not clear (Mee, 2020). Stillbirth is defined as a calving in which the calf is born dead or dies during the first 24 hours after parturition. Other terms like calf livability, perinatal survival, or calf mortality (alive or dead) are also used in addition or instead of stillbirth. In this document we use stillbirth.
Calf mortality may be classified as abortion if it is stillborn before 260 days of gestation, and as stillbirth if it is after 260 days of gestation (Mee, 2020). Calf mortality later than 24 hours after parturition and mortality of young stock will not be considered further in this guideline.
Calving ease is defined as how easy or difficult the calving was. In this document we use calving ease, other terms such as calving difficulty and dystocia are used for similar traits.
Gestation length is the number of days between conception date (usually the last insemination date) and the calving date. Average dairy cattle gestation length is +/- 280 days.
Calf size at birth (or calf birth weight). Often assessed as a subjective score. Calf size is associated with calving ease, stillbirth, and calf mortality. For Holstein the average calf is about 40 kg with a standard deviation of 4 to 5 kg.
Data recording
Registration of calving traits should be done for all calvings within all herds. Calving information is usually recorded by the dairy farmer. In some countries severe cases of dystocia may be recorded via veterinary treatments and be available from health recording system.
Recording of calving traits
The most important traits to record are: Calving ease and stillbirth.
Also recommended: Gestation length and calf size.
Important information for calving traits recording
In general, the following information should be ensured for calving traits:
- Herd ID
- Cow ID
- Parity/lactation number
- Calving date
- ID of calf/calves1
- Sex of calf/calves2
- Number of calves born at calving (twin information)
- Sire ID
- Sire breed
- Calf from embryo? (yes/no); if yes, specify if from Ovum pick up (OPU)
1 ID of calf. From identification & registration perspective all live animals should be identified within 48 hours, but regulations regarding calves born dead may differ between countries. A “dummy” ID needs to be assigned to stillborn calves that have not been assigned an official ID.
2 Sex of calf should always be recorded, as it has a strong influence on calving ease and the importance of including this in the evaluation model increases when sexed semen is used. This also includes the sex of stillborn calves.
Other relevant information for calving traits recording
The following may be useful information related to calving traits:
- Detailed information related to embryo transfer process (see: Section 06 of the ICAR guidelines for recording AI and ET and reporting fertility.
- Calf size
- Insemination dates are needed for calculation of gestation length
- Pelvic area or rump width and rump angle
- Information on sexed semen
Calving Ease scoring scale
The calving ease score should describe how easy or difficult the calving was. The optimum would be to distinguish between the following situations:
- Unassisted unobserved calving (if farmer not present)
- Unassisted observed calving (no assistance needed)
- Easy pull: calving which really needed some manual assistance
- Hard pull: some mechanical assistance required
- Difficult calving: vet assistance required.
- Caesarean section
- Embryotomy
All details may not always be relevant or needed. We recommend that calving ease should be scored in 4 classes. The classes should be well defined and allow easy determination of the class to help keeping accurate records.
- Easy, unassisted: calving without any assistance (also if unobserved/farmer not present)
- Easy pull: calving which really needed some manual assistance
- Difficult calving/Hard pull: some mechanical assistance required, with or without veterinarian aid
- Caesarean section/embryotomy
We recommend that caesarean section and embryotomy be recorded in a separate category, such that these records can easily be omitted when data are used for genetic evaluation.
Other scaling systems exist, and the level of detail needed may vary between breeds and depend on the purpose of data use.
Stillbirth scoring scale
Stillbirth is defined as a calving in which the calf is born dead or dies during the first 24 hours after parturition. We recommend scoring stillbirth using two classes:
- Alive
- Dead at birth or dead within the first 24 hours
Some countries record stillbirth using 3 categories: 1. Alive, 2=Dead at birth, 3=Alive at birth but dead within the first 24 hours.
Calves alive at birth and passing the 24-hour threshold alive must be identified and recorded as such. Therefore, a calf born without information on calf identification and live status should not be assumed to be alive calf.
Recording gestation length
Gestation length is computed from insemination date and calving date (number of days).
Recording calf size
Calf size at birth is often assessed as a subjective score, e.g. small, medium, large. A more accurate alternative would be calf birth weight.
Documentation and data flow
The farmer/dairy producer used to fill in the birth registration for each new born and delivered it to DHI /milk recording organisation. Information related to how the calving took place and on the status of liveability of each calf, was until recently filled in the same form but as optional information, in most countries.
Nowadays, all information related to the calving is becoming more and more relevant, mainly for use in genetic evaluations. As soon as possible after each delivery, calving ease score should be set by the farmer and reported in connection with new born animal id registration, mainly through digital solutions, to assure a complete and an accurate data recording. Digital applications, widely used for animal registration, allowed by different drop-down-menu options recording all information about calving, such as the number of calves born, the sex of each new calf, the size of each new calf and its liveability. For herds without access to digital solutions, information could be recorded by DHI/milk recording technicians or by filling all the information in the traditional registration form and sent it to the correspondent registration organisation within each country.
Data validation
The main issues related with calving traits data recording are:
- Potential under-reporting of dystocia cases: That may result in herds with very low frequency of some calving ease classes.
- Potential misinterpretation of the scale: the differentiation between scores 1 and 2 may not always be well understood. That is why farmers should take into consideration the cow’s needs rather than what they did. For herds with more frequent assisted calving than unassisted calving, scores definition should be discussed with the farmer.
The data validation process has to ensure the usefulness of this information for each purpose and avoid loss of information.
Data validation is generally done in two steps called data verification and data editing.
- Data verification
Basic checks on format and completeness, at the incorporation of data.
For example, Plausibility of ID: animal-ID, herd-ID, calving ease score. Reasonableness of dates: date of insemination, date of calving.
Checking the correctness of data depend on the purpose of use and on the information source.
- Data editing
Data editing should include a clear protocol that describes how to validate the quality of the data from each farm. For calving ease, a check on the distribution of classes is needed. If a herd has a high percentage of records in a single class, the calving ease records from that herd period should be checked with the farmer, and depending on the data uses, they might be omitted.
To define the required period, we should bear in mind that we need to define a minimum number of calving. Depending on the use of the data a minimum frequency could be required.
For genetic evaluation the following edits should be considered:
- If frequency of a single class of calving ease is very low (Less than 1%) it should be combined with the neighbouring class or increased the period. If classes are combined due to the number of cases, data should continuously be carefully monitored. The limits here should follow local circumstances.
- Exclude records of multiple births.
- How to handle calving records resulting from embryo transfer (ET) is a question.
- Exclude all ET records.
- Modelling ET correctly: direct and maternal effects - dam of embryo and cow carrying the calf (recipient cow), pedigree and pe effects
- Include method for ET.
- Breed of sire of calf. How to handle beef on dairy
- Exclude if sire or maternal grandsire of calf is unknown or of another breed.
One solution to these issues is to edit the data used for genetic evaluation and exclude calving records resulting from embryo transfer, records from multiple births (twins), and if sire or maternal grandsire of calf is unknown or of another breed.
For herd management and benchmarking the following edits should be considered:
Data recorded about calving are valuable for herd management and decision-making process. For this use data should be as complete as possible and only records that are completely not consistent with other sources of information such as milk recording data, should be removed.
For benchmarking use, the most important check should be made on the representativeness of the reference group at which belong each record.
Use of data
Routinely recorded calving performance is valuable information that can be used in herd management, documentation of animal welfare, benchmarking and for genetic evaluations.
Genetic evaluation
Model
Ideally, the categorical traits of stillbirth and calving ease should be analyzed using a multivariate threshold model with direct and maternal effects (e.g. Heringstad et al 2007[214]; Cole et al., 2007[215]). However, linear models may often be the model of choice for routine genetic evaluation as they are fast, easy to implement, and in most cases gives a very similar ranking of animals as more advanced models. Eaglen et al. (2012) [216]compared models for calving traits and concluded that multi-trait models had an advantage over univariate models and that extended sire models (i.e. sire maternal grandsire model) are more practical and robust than animal models.
The models used for genetic evaluation must include both direct and maternal effects for all calving traits. Direct effects are the calf’s genetic potential for being born easily and alive, while maternal effects are the cow’s genetic potential for easy calving and liveborn calves
Traits and trait definitions
Precorrection for heterogenous variance may be needed. EuroGenomics (2022) suggest that if a linear model approach is chosen, should approximation to normal distribution using e.g. Snell scores be used (Snell, 1964[217]).
Calving ease is recorded as an ordered categorical trait. How many classes to be used in genetic evaluation is a question. If the frequency is low than 1% in any classes, it may be needed to combine with neighbouring class. However, if the frequency of any class is higher than 90%, the data of the herd-period of time should be eliminated when the aim is estimating breeding values.
In some countries (USA for example) calving ease is defined as calving difficulty expressed as percentage of births of bull calves that are difficult in primiparous heifers and in adult cows.
Calf size and gestation length are examples of genetically correlated traits that may be useful indicator traits to include in a multivariate model together with stillbirth and calving ease.
If multiple parities are included in the genetic evaluation we recommend that first and later parities are treated as genetically correlated trait. Genetic correlations far from 1 suggest that first and later lactation should not be assumed to be the same trait across parities.
Effects to consider
Effects to consider in the model for genetic evaluation of calving traits, in addition to the standard effects such as the cow’s age, contemporary group, and parity, are the sex of calf(s) and the number of calves born (twin information). Calves coming from embryo transfer must be modelled correctly, as a direct effect is coming from the pedigree of the dam that provided the embryo, while the maternal effect (genetic and potentially permanent environment) is coming from the pedigree of the dam that carries the calf.
Consider whether interaction terms to correct for environmental time trends are needed, such as Herd-Year-Age or Herd-Year-Month of calving.
Proofs published
The traits delivered to INTERBULL are only first parity calving traits. It would be an improvement if INTERBULL would allow sending BV predicted for multiple lactations. The traits considered are direct and maternal calving ease and direct and maternal stillbirth. For details related to national genetic evaluations of calving traits see: https://interbull.org/ib/geforms
Calving ease direct: It indicates the influence of the sire on calving ease.
Maternal calving ease: It indicates how easily a sire’s daughter will calve compared to the daughters of other sires.
Breeding values for gestation length and calf size could be useful for herd management purposes.
Genetic parameters
Heritability. The heritabilities of calving performance traits are in general low. The range of heritabilities used for first parity calving traits in national genetic evaluations by countries that deliver calving traits to Interbull are in Table 29 (From: https://interbull.org/ib/geforms), and details are given in Appendix 3: heritability of calving traits used in national genetic evaluations.
| Table 30. Range of heritabilities of calving traits used in national genetic evaluations. | ||||
| Calving Ease | Stillbirth | |||
| Direct | Maternal | Direct | Maternal | |
| Linear model | 0.021 – 0.24 | 0.023 – 0.158 | 0.002 – 0.04 | 0.010 – 0.086 |
| Threshold model | 0.056 – 0.08 | 0.027 - 0.067 | 0.03 - 0.059 | 0.058 - 0.066 |
Genetic correlations. In routine genetic evaluations are the genetic correlation between direct and maternal calving traits often assumed to be zero (https://interbull.org/ib/geforms). Heringstad et al (2007)[218] estimated strong genetic correlations between direct stillbirth and direct calving difficulty (0.79), and between maternal stillbirth and maternal calving difficulty (0.62) for Norwegian Red cows, whereas all genetic correlations between direct and maternal effects within or between traits were close to zero, suggesting that bulls should be evaluated both as sire of calf (direct effect) and sire of the cow (maternal effect).
Herd management use
Information on calving traits are useful in herd management. Farmers try to consider an endless list of best practices and recommended standards to ensure a good preparation for calving. Nevertheless, there is no clear evidence of their effectiveness. On the other hand, it is known that herd management to reduce dystocia cases should start with heifers’ development.
The best way to know if something is going wrong around calving within a specific farm is by using calving ease scores and monitoring the situation over different periods of time. Reducing the number of dystocia cases will improve cow- as well as calf health and animal welfare. Examples on measures that can improve calving performance:
- Make breeding plans to avoid difficult calvings. Consider the bulls breeding value for calving ease and calf size (direct effect, sire of calf) when choosing which bulls to use for each cow. Avoid using bulls that gives large calves to heifers/small cows and to cows that had difficult calving in the past (e.g. GENEX, 2022[219]).
- Breeding values for gestation length (direct effect, sire of calf) can be used to predict expected calving date more accurately and thereby be an useful herd management tool.
- Use information on calving performance when making culling decisions for the herd.
Unfortunately, evidence-based best management practices for animals around calving are largely unknown, with several knowledge gaps still existing on the subject. Further investigations on the effect of management practices, on the effect of environmental conditions on calving time, and on cow-calving behaviours are needed to understand better calving process and help farmers with more information about how to improve dairy cow’s management around calving period. Meanwhile, analysing, throughout seasons/years of calving, the easy-calving-score frequencies to detect any issues and check all risk factors to find out their grounds.
Animal welfare use
Ensuring a high animal welfare on dairy industry may rely on many factors, which could be related to herd management, farm facilities and animal abilities. The objective way to assess animal welfare should be related to animal performances. Calving performance traits, considered as health or reproductive aspects by animal welfare expert, are ones of the important performances taken account by animal welfare protocol assessments. Routinely recorded herd data, such as records on stillbirths and dystocia, can be used for documentation of animal welfare status (Haskell et al. 2019[220]; OIE, 2020[221]).
Acknowledgements
We are grateful to EuroGenomics, who shared their knowledge and experience, and gave access to their document “Golden Standard for calving traits (https://www.eurogenomics.com/golden-standards.html), which aim at harmonization of traits within the EuroGenomics collaboration.
Appendix 3: Heritability of calving traits used in national genetic evaluations.
| Table 1. Heritability of calving traits used in national genetic evaluations by countries that deliver calving traits to Interbull (from: https://interbull.org/ib/geforms, accessed March 2022). | ||||||
| Country | Breed1 | Model2 | Calving Ease | Stillbirth | ||
| Direct | Maternal | Direct | Maternal | |||
| Australia | HOL | MT AM | 0.07 | |||
| Belgium | HOL | ST AM | 0.077 | 0.023 | ||
| Canada | HOL, BWS, GUE | MT AM | 0.036 | 0.125 | 0.0055 | 0.071 |
| AYR | MT AM | 0.048 | 0.086 | 0.004 | 0.06 | |
| JER | MT AM | 0.021 | 0.158 | 0.0018 | 0.0712 | |
| Denmark, Finland, Sweden | HOL | MT AM | 0.08 | 0.06 | 0.04 | 0.035 |
| RDC | MT AM | 0.06 | 0.04 | 0.035 | 0.02 | |
| France | HOL | THR | 0.056 | 0.032 | 0.03 | 0.066 |
| BSW | THR | 0.074 | 0.043 | 0.059 | 0.058 | |
| Germany, Austria, Luxemburg | HOL | MT AM | 0.048 | 0.039 | 0.027 | 0.054 |
| Austria, Germany | BSW | MT AM | 0.057 | 0.065 | 0.013 | 0.010 |
| Austria, Germany, Czech Republic | FL | MT AM | 0.066 | 0.105 | 0.012 | 0.011 |
| GBR | HOL | S-MGS | 0.068 | 0.044 | ||
| Hungary | HOL | MT AM | 0.24 | 0.156 | ||
| Ireland | HOL, RDC | AM | 0.09 | |||
| Israel | HOL | MT AM | 0.06 | 0.03 | 0.03 | 0.014 |
| Italia | HOL | THR | 0.08 | 0.036 | ||
| Netherlands | All | MT AM | 0.068 | 0.048 | 0.038 | 0.086 |
| New Zeeland | All | MT AM | 0.045 | |||
| Norway | RDC | MT AM | 0.068 | 0.048 | 0.011 | 0.011 |
| Poland | HOL | MT AM | 0.048 | 0.039 | 0.027 | 0.054 |
| Slovakia | HOL | THR, S-MGS | 0.072 | 0.067 | ||
| Spain | HOL | THR, S-MGS | 0.072 | 0.027 | ||
| Switzerland | HOL | MT, S-MGS | 0.053 | 0.041 | 0.007 | 0.02 |
| USA | HOL | THR, S-MGS | 0.072 | 0.053 | 0.03 | 0.065 |
1Breed: HOL=Holstein, RDC=Red Dairy Cattle, AYR=Ayrshire, JER=Jersey; FL=Fleckvieh.
2MT=multi-trait model, AM=animal model, S-MGS=Sire maternal grandsire, THR=Threshold model.
- ↑ European Commission, 2007: European Union Animal Health Strategy (2007-2013): prevention is better than cure. http://ec.europa.eu/food/animal/diseases/strategy/animal_health_strategy_en.pdf
- ↑ Nielsen, U. S., Aamand, G. P., Mark, T., 2000. National genetic evaluation of udder health and other traits in Denmark. Interbull Open Meeting, Bled, 2000, Interbull Bulletin 25: 143‑150.
- ↑ Phillipson, J., Lindhe, B., 2003. Experiences of including reproduction and health traits in Scandinavian dairy cattle breeding programmes. Livestock Production Sci. 83: 99-112.
- ↑ Østerås, O., Sølverød, L., 2005. Mastitis control systems: the Norwegian experience. In: Hogevven, H. (Ed.), Mastitis in dairy production: Current knowledge and future solutions, Wageningen Academic Publishers, The Netherlands, 91-101.
- ↑ Aamand, G. P., 2006. Data collection and genetic evaluation of health traits in the Nordic countries. British Cattle Conference, Shrewsbury, UK, 2006.
- ↑ Heringstad, B., Klemetsdal, G., Steine, T., 2007. Selection responses for disease resistance in two selection experiments with Norwegian red cows. J. Dairy Sci. 90: 2419-2426.
- ↑ Zwald, N. R., Weigel, K. A., Chang, Y. M., Welper R. D., Clay, J. S., 2004a. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates and sire breeding values. J. Dairy Sci. 87: 4287-4294.
- ↑ Zwald, N. R., Weigel, K. A., Chang, Y. M., Welper R. D., Clay, J. S., 2004b. Genetic selection for health traits using producer-recorded data. II. Genetic correlations, disease probabilities and relationships with existing traits. J. Dairy Sci. 87: 4295-4302.
- ↑ Neuenschwander, T. F.-O., Miglior, F., Jamrocik, J., Schaeffer, L. R., 2008. Comparison of different methods to validate a dataset with producer-recorded health events. http://cgil.uoguelph.ca/dcbgc/Agenda0809/Health_180908.pdf
- ↑ Neuenschwander, T.F.O., 2010. Studies on disease resistance based on producer-recorded data in Canadian Holsteins. PhD thesis. University of Guelph, Guelph, Canada.
- ↑ Appuhamy, J.A.D.R.N., Cassell, B.G., Cole, J.B., 2009. Phenotypic and genetic relationship of common health disorders with milk and fat yield persistencies from producer-recorded health data and test-day yields. J. Dairy Sci. 92: 1785-1795.
- ↑ Egger-Danner, C., Obritzhauser, W., Fuerst-Waltl, B., Grassauer, B., Janacek, R., Schallerl, F., Litzllachner, C., Koeck, A., Mayerhofer, M., Miesenberger J., Schoder, G., Sturmlechner, F., Wagner, A., Zottl, K., 2010. Registration of health traits in Austria - experience review. Proc. ICAR 37th Annual Meeting - Riga, Latvia. 31.5. - 4.6. 2010.
- ↑ Egger-Danner, C., Fuerst-Waltl, B., Obritzhauser, W., Fuerst, C., Schwarzenbacher, H., Grassauer, B., Mayerhofer, M., Koeck, A., 2012. Recording of direct health traits in Austria - experience report with emphasis on aspects of availability for breeding purposes. J. Dairy Sci. (in press).
- ↑ Koeck, A., F. Miglior, D. F. Kelton, and F. S. Schenkel (2012a). Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J. Dairy Sci. 95:432-439.
- ↑ Koeck, A., F. Miglior, D. F. Kelton, and F. S. Schenkel (2012b). Health recording in Canadian Holsteins - data and genetic parameters. J. Dairy Sci. (submitted for publication). LeBlanc, S. J., Lissemore, K. D., Kelton, D. F., Duffield, T. F., Leslie, K. E., 2006. Major advances in disease prevention in dairy cattle.J. Dairy Sci. 89:1267-1279
- ↑ Neuenschwander, T. F.-O., F. Miglior, J. Jamrozik, O. Berke, D. F. Kelton, and L. Schaeffer. 2012. Genetic parameters for producer-recorded health data in Canadian Holstein cattle. Animal DOI: 10.1017/S1751731111002059.
- ↑ Kelton, D. F., Lissemore, K. D., Martin. R. E., 1998. Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J. Dairy Sci. 81: 2502-2509.
- ↑ Cole, J.B., Sanders, A.H., and Clay, J.S., 2006: Use of producer-recorded health data in determining incidence risks and relationships between health events and culling. J. Dairy Sci. 89(Suppl. 1):10(abstr. M7).
- ↑ Soyeurt, H., Dardenne, P., Gengler, N, 2009a. Detection and correction of outliers for fatty acid contents measured by mid-infrared spectrometry using random regression test-day models. 60th Annual Meeting of the EAAP, Barcelona 24-27, 2009, Spain.
- ↑ Soyeurt, H., Arnould, V.M.-R., Dardenne, P., Stoll, J., Braun, A., Zinnen, Q., Gengler, N. 2009b. Variability of major fatty acid contents in Luxembourg dairy cattle.60th Annual Meeting of the EAAP, Barcelona 24-27, 2009, Spain.
- ↑ Zwald, N. R., Weigel, K. A., Chang, Y. M., Welper R. D., Clay, J. S., 2004a. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates and sire breeding values. J. Dairy Sci. 87: 4287-4294.
- ↑ Østerås, O., Solbu, H., Refsdal, A. O., Roalkvan, T., Filseth, O., Minsaas, A., 2007. Results and evaluation of thirty years of health recordings in the Norwegian dairy cattle population. J. Dairy Sci. 90: 4483-4497.
- ↑ Østerås, O. 2012. Årsrapport Helsekortordningen 2011.pdf. http://storfehelse.no/6689.cms . Accessed, April 16, 2012.
- ↑ Capion, N., Thamsborg, S.M.,Enevoldsen, C., 2008. Prevalence of foot lesions in Danish Holstein cows. Veterinary Record 2008, 163:80-96.
- ↑ Thomsen, P.T., Klaas, I.C. and Bach, K., 2008. Short communication: scoring of digital dermatitis during milking as an alternative to scoring in a hoof trimming chute. J. Dairy Sci. 91:4679-4682.
- ↑ Maier, M., 2009. Erfassung von Klauenveränderungen im Rahmen der Klauenpflege. Diplomarbeit, Universität für Bodenkultur, Vienna.
- ↑ Buch, L.H., Sorensen, A.C., Lassen, J., Berg, P., Eriksson, J-.A., Jakobsen, J.H., Sorensen, M.K., 2011. Hygiene-related and feed-related hoof diseases show different patterns of genetic correlations to clinical mastitis and female fertility. J. Dairy Sci. 94:1540-1551.
- ↑ Neuenschwander, T. F.-O., Miglior, F., Jamrocik, J., Schaeffer, L. R., 2008. Comparison of different methods to validate a dataset with producer-recorded health events. http://cgil.uoguelph.ca/dcbgc/Agenda0809/Health_180908.pdf
- ↑ USDA, 2010. Format 6, the data exchange format health events. http://aipl.arsusda.gov/CFRCS/GetRCS.cfm?DocType=formats&DocName=fmt6.html
- ↑ Neuenschwander, T. F.-O., Miglior, F., Jamrocik, J., Schaeffer, L. R., 2008. Comparison of different methods to validate a dataset with producer-recorded health events. http://cgil.uoguelph.ca/dcbgc/Agenda0809/Health_180908.pdf
- ↑ USDA, 2010. Format 6, the data exchange format health events. http://aipl.arsusda.gov/CFRCS/GetRCS.cfm?DocType=formats&DocName=fmt6.html
- ↑ Kelton, D. F., Lissemore, K. D., Martin. R. E., 1998. Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J. Dairy Sci. 81: 2502-2509.
- ↑ Heringstad, B., Klemetsdal, G., Steine, T., 2007. Selection responses for disease resistance in two selection experiments with Norwegian red cows. J. Dairy Sci. 90: 2419-2426.
- ↑ Koeck, A., F. Miglior, D. F. Kelton, and F. S. Schenkel (2012a). Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J. Dairy Sci. 95:432-439.
- ↑ Koeck, A., F. Miglior, D. F. Kelton, and F. S. Schenkel (2012b). Health recording in Canadian Holsteins - data and genetic parameters. J. Dairy Sci. (submitted for publication). LeBlanc, S. J., Lissemore, K. D., Kelton, D. F., Duffield, T. F., Leslie, K. E., 2006. Major advances in disease prevention in dairy cattle.J. Dairy Sci. 89:1267-1279
- ↑ Wolff, C., 2012. Validation of the Nordic Disease Recording Systems for Dairy Cattle with Special Reference to Clinical Mastitis. Doctoral Thesis. Faculty of Veterinary Medicine and Animal Science, Department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala 2012. http://pub.epsilon.slu.se/8546/1/wolff_c_120110.pdf
- ↑ Aamand, G. P., 2006. Data collection and genetic evaluation of health traits in the Nordic countries. British Cattle Conference, Shrewsbury, UK, 2006.
- ↑ Heringstad, B., Klemetsdal, G., Steine, T., 2007. Selection responses for disease resistance in two selection experiments with Norwegian red cows. J. Dairy Sci. 90: 2419-2426.
- ↑ Wolff, C., 2012. Validation of the Nordic Disease Recording Systems for Dairy Cattle with Special Reference to Clinical Mastitis. Doctoral Thesis. Faculty of Veterinary Medicine and Animal Science, Department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala 2012. http://pub.epsilon.slu.se/8546/1/wolff_c_120110.pdf
- ↑ USDA, 2010. Format 6, the data exchange format health events. http://aipl.arsusda.gov/CFRCS/GetRCS.cfm?DocType=formats&DocName=fmt6.html
- ↑ Egger-Danner, C., Fuerst-Waltl, B., Janacek, R., Mayerhofer, M., Obritzhauser, W., Reith, F., Tiefenthaller, F., Wagner, A., Winter, P., Wöckinger, M., Wurm, K., Zottl, K., 2007. Sustainable cattle breeding supported by health reports. 58th Annual Meeting of the EAAP, August 26-29, 2007, Dublin.
- ↑ Schwarzenbacher, H., Obritzhauser, W., Fuerst-Waltl, B., Koeck, A., Egger-Danner, C., 2010. Health monitoring yystem in Austrian dual purpose Fleckvieh cattle: incidences and prevalences. In: EAAP-Book of Abstracts No 11: 61th Annual Meeting of the EAAP, August 23-27, 2010 Heraklion, Greece.
- ↑ Ducrocq, V., 2010: Sustainable dairy cattle breeding: illusion or reality? 9th World Congress on Genetics Applied to Livestock Production. 1.-6.8.2010, Leipzig, Germany.
- ↑ Heringstad, B., Klemetsdal, G., Steine, T., 2007. Selection responses for disease resistance in two selection experiments with Norwegian red cows. J. Dairy Sci. 90: 2419-2426.
- ↑ Østerås, O., Solbu, H., Refsdal, A. O., Roalkvan, T., Filseth, O., Minsaas, A., 2007. Results and evaluation of thirty years of health recordings in the Norwegian dairy cattle population. J. Dairy Sci. 90: 4483-4497.
- ↑ Johansson, K., S. Eriksson, J. Pösö, M. Toivonen, U. S. Nielsen, J.A. Eriksson, G.P. Aamand. 2006. Genetic evaluation of udder health traits for Denmark, Finland and Sweden. Interbull Bulletin 35: 92-96.
- ↑ Johansson, K., J. Pöso, U. S. Nielsen, J.A.Eriksson, G.P. Aamand., 2008. Joint genetic evaluation of other disease traits in Denmark, Finland and Sweden. Interbull Meeting, Interbull Bulletin 38:107-112.
- ↑ Interbull, 2010. Description of GES as applied in member countries. http://www-interbull.slu.se/national_ges_info2/framesida-ges.htm
- ↑ Negussie, M., M. Lidauer, E.A. Mäntysaari, I. Stranden, J. Pösö, U.S. Nielsen, K. Johansson, J-A. Eriksson, G.P. Aamand. 2010. Combining test day SCS with clinical mastitis and udder type traits: a random regression model for joint genetic evaluation of udder health in Denmark, Finland and Sweden. Interbull Bulletin 42: 25-31.
- ↑ Aamand, G. P., 2006. Data collection and genetic evaluation of health traits in the Nordic countries. British Cattle Conference, Shrewsbury, UK, 2006.
- ↑ Zwald, N. R., Weigel, K. A., Chang, Y. M., Welper R. D., Clay, J. S., 2004a. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates and sire breeding values. J. Dairy Sci. 87: 4287-4294.
- ↑ Zwald, N. R., Weigel, K. A., Chang, Y. M., Welper R. D., Clay, J. S., 2004b. Genetic selection for health traits using producer-recorded data. II. Genetic correlations, disease probabilities and relationships with existing traits. J. Dairy Sci. 87: 4295-4302.
- ↑ Heringstad, B., Klemetsdal, G., Steine, T., 2007. Selection responses for disease resistance in two selection experiments with Norwegian red cows. J. Dairy Sci. 90: 2419-2426.
- ↑ Koeck, A., Heringstad, B., Egger-Danner, C., Fuerst, C., Fuerst-Waltl, B., 2010. Comparison of different models for genetic analysis of clinical mastitis in Austrian Fleckvieh dual purpose cows. J. Dairy Sci. (in press).
- ↑ Koeck, A., F. Miglior, D. F. Kelton, and F. S. Schenkel (2012a). Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J. Dairy Sci. 95:432-439.
- ↑ Zwald, N. R., Weigel, K. A., Chang, Y. M., Welper R. D., Clay, J. S., 2004a. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates and sire breeding values. J. Dairy Sci. 87: 4287-4294.
- ↑ Zwald, N. R., Weigel, K. A., Chang, Y. M., Welper R. D., Clay, J. S., 2004a. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates and sire breeding values. J. Dairy Sci. 87: 4287-4294.
- ↑ Heringstad, B., Chang, Y.M., Gianola, D., Klemetsdal, G., 2005. Genetic correlations between clinical mastitis, milk fever, ketosis and retained placenta within and between the first three lactations of Norwegian Red (NRF). In: EAAP-Book of Abstracts No 11: 56th Annual Meeting of the EAAP, 3-4.6..2005 Uppsala, Sweden.
- ↑ Johansson, K., S. Eriksson, J. Pösö, M. Toivonen, U. S. Nielsen, J.A. Eriksson, G.P. Aamand. 2006. Genetic evaluation of udder health traits for Denmark, Finland and Sweden. Interbull Bulletin 35: 92-96.
- ↑ Johansson, K., J. Pöso, U. S. Nielsen, J.A.Eriksson, G.P. Aamand., 2008. Joint genetic evaluation of other disease traits in Denmark, Finland and Sweden. Interbull Meeting, Interbull Bulletin 38:107-112.
- ↑ Negussie, M., M. Lidauer, E.A. Mäntysaari, I. Stranden, J. Pösö, U.S. Nielsen, K. Johansson, J-A. Eriksson, G.P. Aamand. 2010. Combining test day SCS with clinical mastitis and udder type traits: a random regression model for joint genetic evaluation of udder health in Denmark, Finland and Sweden. Interbull Bulletin 42: 25-31.
- ↑ Pritchard, T.C., R. Mrode, M.P. Coffey, E. Wall., 2011. Combination of test day somatic cell count and incidence of mastitis for the genetic evaluation of udder health. Interbull-Meeting. Stavanger, Norway. http://www.interbull.org/images/stories/Pritchard.pdf . Accessed November 2, 2011.
- ↑ Urioste, J.I., J. Franzén, J.J.Windig, E. Strandberg., 2011. Genetic variability of alternative somatic cell count traits and their relationship with clinical and subclinical mastitis. Interbull-meeting. Stavanger, Norway. http://www.interbull.org/images/stories/Urioste.pdf . Accessed November 2, 2011.
- ↑ Koeck, A., F. Miglior, D. F. Kelton, and F. S. Schenkel (2012a). Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J. Dairy Sci. 95:432-439.
- ↑ Koeck, A., F. Miglior, D. F. Kelton, and F. S. Schenkel (2012b). Health recording in Canadian Holsteins - data and genetic parameters. J. Dairy Sci. (submitted for publication). LeBlanc, S. J., Lissemore, K. D., Kelton, D. F., Duffield, T. F., Leslie, K. E., 2006. Major advances in disease prevention in dairy cattle.J. Dairy Sci. 89:1267-1279
- ↑ Nielsen, U. S., Aamand, G. P., Mark, T., 2000. National genetic evaluation of udder health and other traits in Denmark. Interbull Open Meeting, Bled, 2000, Interbull Bulletin 25: 143‑150.
- ↑ Nielsen, U. S., Aamand, G. P., Mark, T., 2000. National genetic evaluation of udder health and other traits in Denmark. Interbull Open Meeting, Bled, 2000, Interbull Bulletin 25: 143‑150.
- ↑ Nielsen, U. S., Aamand, G. P., Mark, T., 2000. National genetic evaluation of udder health and other traits in Denmark. Interbull Open Meeting, Bled, 2000, Interbull Bulletin 25: 143‑150.
- ↑ Heringstad, B., Chang, Y.M., Gianola, D., Klemetsdal, G., 2005. Genetic correlations between clinical mastitis, milk fever, ketosis and retained placenta within and between the first three lactations of Norwegian Red (NRF). In: EAAP-Book of Abstracts No 11: 56th Annual Meeting of the EAAP, 3-4.6..2005 Uppsala, Sweden.
- ↑ Negussie, M., M. Lidauer, E.A. Mäntysaari, I. Stranden, J. Pösö, U.S. Nielsen, K. Johansson, J-A. Eriksson, G.P. Aamand. 2010. Combining test day SCS with clinical mastitis and udder type traits: a random regression model for joint genetic evaluation of udder health in Denmark, Finland and Sweden. Interbull Bulletin 42: 25-31.
- ↑ Koeck, A., Egger-Danner, C., Fuerst, C., Obritzhauser, W., Fuerst-Waltl, B., 2010. Genetic analysis of reproductive disorders and their relationship to fertility and milk yield in Austrian Fleckvieh dual purpose cows. J. Dairy Sci. 93: 2185-2194.
- ↑ Koeck, A., Egger-Danner, C., Fuerst, C., Obritzhauser, W., Fuerst-Waltl, B., 2010. Genetic analysis of reproductive disorders and their relationship to fertility and milk yield in Austrian Fleckvieh dual purpose cows. J. Dairy Sci. 93: 2185-2194.
- ↑ Koeck, A., Egger-Danner, C., Fuerst, C., Obritzhauser, W., Fuerst-Waltl, B., 2010. Genetic analysis of reproductive disorders and their relationship to fertility and milk yield in Austrian Fleckvieh dual purpose cows. J. Dairy Sci. 93: 2185-2194.
- ↑ Koeck, A., L. R. Schenkel, G. J. Kistner, C. Egger-Danner, and F. S. Miglior. 2010. Genetic analysis of clinical mastitis and its relationship with somatic cell score and milk production in first lactation Canadian Jersey cows. J. Dairy Sci. 93: 4355-4363.
- ↑ Pryce, J.E. & Veerkamp R.F., 1999. The incorporation of fertility indices in genetic improvement programmes. Br. Soc. Anim;Vol 1:Occasional Mtg. Pub. 26.
- ↑ Sun, C., Madsen, P., Lund M.S., Zhang Y, Nielsen U.S. & Su S., 2010. Improvement in genetic evaluation of female fertility in dairy cattle using multiple-trait models including milk production traits. J. Anim. Sci. 88:871-878.
- ↑ Bastin, C., Soyeurt, H., Vanderick, S. & Gengler, N., 2011. Genetic relationships between milk fatty acids and fertility of dairy cows. Interbull Bulletin 44, 190-194.
- ↑ Veerkamp, R. F., Koenen, E. P. C. & De Jong, G. 2001. Genetic correlations among body condition score, yield, and fertility in first-parity cows estimated by random regression models. J. Dairy Sci. 84, 2327-2335.
- ↑ Harris, B.L., Pryce, J.E. & Montgomerie, W.A., 2007. Experiences from breeding for economic efficiency in dairy cattle in New Zealand Proc. Assoc. Advmt. Anim. Breed. Genet. 17:434.
- ↑ Gredler, B. Fuerst, C. & Soelkner, H., 2007. Analysis of New Fertility Traits for the Joint Genetic Evaluation in Austria and Germany. Interbull Bulletin 37, 152-155.
- ↑ Breen, J.E., Hudson, C.D., Bradley, A.J. & Green, M.J., 2009. Monitoring dairy herd fertility performance in the modern production animal practice. British Cattle Veterinary Association (BCVA) Congress, Southport, November 2009.
- ↑ Austrian Ministry of Health, 2010. Kundmachung des TGD-Programms Gesundheitsmonitoring Rind. http://bmg.gv.at/home/Schwerpunkte/Tiergesundheit/Rechtsvorschriften/Kundmachungen/Kundmachung_des_TGD_Programms_Gesundheitsmonitoring_Rind.
- ↑ Breen, J.E., Hudson, C.D., Bradley, A.J. & Green, M.J., 2009. Monitoring dairy herd fertility performance in the modern production animal practice. British Cattle Veterinary Association (BCVA) Congress, Southport, November 2009.
- ↑ Austrian Ministry of Health, 2010. Kundmachung des TGD-Programms Gesundheitsmonitoring Rind. http://bmg.gv.at/home/Schwerpunkte/Tiergesundheit/Rechtsvorschriften/Kundmachungen/Kundmachung_des_TGD_Programms_Gesundheitsmonitoring_Rind.
- ↑ Johansson, K., J.-Å. Eriksson, U.S. Nielsen, J. Pösö, and G.P. Aamand. 2011. Genetic evaluation of claw health in Denmark, Finland and Sweden. Interbull Bull. 44:224–228.
- ↑ Ødegård, C., M. Svendsen, and B. Heringstad. 2013. Genetic analyses of claw health in Norwegian Red cows. J. Dairy Sci. 96:7274–7283. doi:10.3168/jds.2012-6509.
- ↑ 87.0 87.1 Häggman, J., and J. Juga. 2013. Genetic parameters for hoof disorders and feet and leg conformation traits in Finnish Holstein cows. J. Dairy Sci. 96:3319–3325. doi:10.3168/jds.2012-6334.
- ↑ Kofler, J., 2013. Computerised claw trimming database programs as the basis for monitoring hoof health in dairy herds. Veterinary Journal 198, 358-361.
- ↑ ICAR Claw Health Atlas
- ↑ Egger-Danner, C., P. Nielsen, A. Fiedler, A. Müller, T. Fjeldaas, D. Döpfer, V. Daniel, C. Bergsten, G. Cramer, A.-M. Christen, K.F. Stock, G. Thomas, M. Holzhauer, A. Steiner, J. Clarke, N. Capion, N. Charfeddine, J.E. Pryce, E. Oakes, J. Burgstaller, B. Heringstad, C. Ødegård, J. Kofler, F. Egger, and J.B. Cole. 2015. ICAR Claw Health Atlas. ICAR Technical Series. No. 18. International Committee for Animal Recording, Rome, Italy.
- ↑ Koenig, S., A.R. Sharifi, H. Wentrot, D. Landmann, M. Eise, and H. Simianer. 2005. Genetic parameters of claw and foot disorders estimated with logistic models. J. Dairy Sci. 88:3316–3325. doi:10.3168/jds.S0022-0302 (05)73015-0.
- ↑ Van Pelt, M.L. 2015. Implementation of a claw health index in The Netherlands. Page Seminar des Ausschusses für Genetik der ZAR, Salzburg, Austria.
- ↑ Aamand, G.P. 2006. Data collection and genetic evaluation of health traits in the Nordic countries. Page British Cattle Breeders Conference, Shrewsbury, UK.
- ↑ Egger-Danner, C., B. Fuerst-Waltl, W. Obritzhauser, C. Fuerst, H. Schwarzenbacher, B. Grassauer, M. Mayerhofer, and A. Koeck. 2012. Recording of direct health traits in Austria—Experience report with emphasis on aspects of availability for breeding purposes. J. Dairy Sci. 95:2765–2777. doi:10.3168/jds.2011-4876.
- ↑ Østerås, O., H. Solbu, A.O. Refsdal, T. Roalkvam, O. Filseth, and A. Minsaas. 2007. Results and evaluation of thirty years of health recordings in the Norwegian dairy cattle population. J. Dairy Sci. 90:4483–4497. doi:10.3168/jds.2007-0030.
- ↑ Sprecher, D.J., D.E. Hostetler, and J.B. Kaneene. 1997. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 47:1179–1187. doi:10.1016/S0093-691X(97)00098-8.
- ↑ Flower, F.C., and D.M. Weary. 2006. Effect of hoof pathologies on subjective assessments of dairy cow gait. J. Dairy Sci. 89:139–146. doi:10.3168/jds.S0022-0302(06)72077-X.
- ↑ Berry, S.L., D.H. Read, R.L. Walker, and T.R. Famula. 2010. Clinical, histologic, and bacteriologic findings in dairy cows with digital dermatitis (footwarts) one month after topical treatment with lincomycin hydrochloride or oxytetracycline hydrochloride. J. Am. Vet. Med. Assoc. 237:555–560. doi:10.2460/javma.237.5.555.
- ↑ Gaddis, K.L.P., J.B. Cole, J.S. Clay, and C. Maltecca. 2014. Genomic selection for producer-recorded health event data in US dairy cattle. J. Dairy Sci. 97:3190–3199. doi:10.3168/jds.2013-7543.
- ↑ Koeck, A., S. Loker, F. Miglior, D.F. Kelton, J. Jamrozik, and F.S. Schenkel. 2014. Genetic relationships of clinical mastitis, cystic ovaries, and lameness with milk yield and somatic cell score in first-lactation Canadian Holsteins. J. Dairy Sci. 97:5806–5813. doi:10.3168/jds.2013-7785.
- ↑ Egger-Danner, C., A. Koeck, J. Kofler, J. Burgstaller, F. Steininger, C. Fuerst, and B. Fürst-Waltl. 2017. Evaluation of different data sources for genetic improvement of claw health in Austrian Fleckvieh (Simmental) and Brown Swiss cattle. Proceedings of the 19th International Symposium and 11th International Conference on Lameness in Ruminants, 6-9 Sep, 2017, Munich, Germany.
- ↑ Tomlinson, D.J., C.H. Mülling, and T.M. Fakler. 2004. Invited Review: Formation of keratins in the bovine claw: roles of hormones, minerals, and vitamins in functional claw integrity. J. Dairy Sci. 87:797–809. doi:10.3168/jds.S0022-0302 (04)73223-3Van der Linde, C., G. de Jong, E.P.C. Koenen, and H. Eding. 2010. Claw health index for Dutch dairy cattle based on claw trimming and conformation data. J. Dairy Sci. 93:4883–4891. doi:10.3168/jds.2010-3183.
- ↑ Tadich, N., E. Flor, and L. Green. 2010. Associations between hoof lesions and locomotion score in 1098 unsound dairy cows. Vet. J. 184:60–65. doi:10.1016/j.tvjl.2009.01.005.
- ↑ Bicalho, R.C., and G. Oikonomou. 2013. Control and prevention of lameness associated with claw lesions in dairy cows. Livest. Sci. 156:96–105. doi:10.1016/j.livsci.2013.06.007.
- ↑ Alsaaod, M., C. Syring, M. Luternauer, M. G. Doherr and A. Steiner, 2015. Effect of routine claw trimming on claw temperature in dairy cows measured by infrared thermography. J. Dairy Sci. 98:2381–2388.
- ↑ Beer, G., M. Alsaaod, A. Starke, G. Schuepbach-Regula, H. Müller, P. Kohler, and A. Steiner. 2016. Use of extended characteristics of locomotion and feeding behavior for automated identification of lame dairy cows. PLOS ONE 11:e0155796.
- ↑ Nechanitzky, K., A. Starke, B. Vidondo, H. Müller, M. Reckardt, K. Friedli, and A. Steiner. 2016. Analysis of behavioral changes in dairy cows associated with claw horn lesions. J. Dairy Sci. 99:2904–2914. doi:10.3168/jds.2015-10109.
- ↑ Beer, G., M. Alsaaod, A. Starke, G. Schuepbach-Regula, H. Müller, P. Kohler, and A. Steiner. 2016. Use of extended characteristics of locomotion and feeding behavior for automated identification of lame dairy cows. PLOS ONE 11:e0155796.
- ↑ 109.0 109.1 109.2 Egger-Danner, C., P. Nielsen, A. Fiedler, A. Müller, T. Fjeldaas, D. Döpfer, V. Daniel, C. Bergsten, G. Cramer, A.-M. Christen, K.F. Stock, G. Thomas, M. Holzhauer, A. Steiner, J. Clarke, N. Capion, N. Charfeddine, J.E. Pryce, E. Oakes, J. Burgstaller, B. Heringstad, C. Ødegård, J. Kofler, F. Egger, and J.B. Cole. 2015. ICAR Claw Health Atlas. ICAR Technical Series. No. 18. International Committee for Animal Recording, Rome, Italy.
- ↑ 110.0 110.1 110.2 Kofler, J., J.A. Hangl, R. Pesenhofer, and G. Landl. 2011. Evaluation of claw health in heifers in seven dairy farms using a digital claw trimming protocol and claw data analysis system. Berl Münch Tierärztl Wochenschr 124:272–281.
- ↑ Kofler, J., 2013. Computerised claw trimming database programs as the basis for monitoring hoof health in dairy herds. Veterinary Journal 198, 358-361.
- ↑ Nielsen, P. 2014. Claw health data – recording and usage in Denmark. Page in ICAR Technical Series no. 18 39th ICAR Biennial Session. International Committee for Animal Recording, Rome, Italy, Berlin, Germany.
- ↑ Van Pelt, M.L. 2015. Implementation of a claw health index in The Netherlands. Page Seminar des Ausschusses für Genetik der ZAR, Salzburg, Austria.
- ↑ Kofler, J., J.A. Hangl, R. Pesenhofer, and G. Landl. 2011. Evaluation of claw health in heifers in seven dairy farms using a digital claw trimming protocol and claw data analysis system. Berl Münch Tierärztl Wochenschr 124:272–281.
- ↑ Dopfer, 2009. Digital Dermatitis The dynamics of digital dermatitis in dairy cattle and the manageable state of disease. CanWest Conference October 17 – 20, 2009. http://hoofhealth.ca/Dopfer.pdf.
- ↑ Pérez-Cabal, M.A., and N. Charfeddine. 2015. Models for genetic evaluations of claw health traits in Spanish dairy cattle. J. Dairy Sci. 98: 8186-8194. doi:10.3168/jds.2015-9562.
- ↑ Van der Spek, D., J.A.M. van Arendonk, A.A.A. Vallée, and H. Bovenhuis. 2013. Genetic parameters for claw disorders and the effect of preselecting cows for trimming. J. Dairy Sci. 96:6070–6078. doi:10.3168/jds.2013-6833.
- ↑ Van der Waaij, E.H., M. Holzhauer, E. Ellen, C. Kamphuis, and G. de Jong. 2005. Genetic parameters for claw disorders in Dutch dairy cattle and correlations with conformation traits. J. Dairy Sci. 88:3672–3678. doi:10.3168/jds.S0022-0302(05)73053-8.
- ↑ Veerkamp, R.F., Gerritsen, C. L. M., Koenen, E. P. C. , Hamoen, A., and De Jong, G. 2002. Evaluation of Classifiers that Score Linear Type Traits and Body Condition Score Using Common Sires. J. Dairy Sci. 85:976–983
- ↑ Dohoo I., Martin W. and Stryhn H. 2009. Veterinary Epidemiologic research. 2nd Edition. Published by VER Inc. Canada.
- ↑ Cramer, G. & C. Guard, 2011. Recommendations for the calculation of incidence rates for monitoring foot health. Proceedings of the 16th International Symposium & 8th Conference on Lameness in Ruminants, New Zealand.
- ↑ Von Keyserlingk, M.A.G., Barrientos, A., Ito, K., Galo, E., and Weary, D,M. 2012. Benchmarking cow comfort on North American freestall dairies: Lameness, leg injuries, lying time, facility design, and management for high-producing Holstein dairy cows. Journal of Dairy Science 95:7399–7408.
- ↑ Bradley, A. J., J. E. Breen, C. D. Hudson, and M. J. Green. 2013. Benchmarking for health from the perspective of consultants. ICAR Technical Meeting Aarhus (Denmark), 29 – 31 May 2013. http://www.icar.org/index.php/icar-meetings-news/aarhus-2013
- ↑ Dohoo I., Martin W. and Stryhn H. 2009. Veterinary Epidemiologic research. 2nd Edition. Published by VER Inc. Canada.
- ↑ Heringstad, B., C. Egger-Danner, N. Charfeddine, J.E. Pryce, K.F. Stock, J. Kofler, A.M. Sogstad, M. Holzhauer, A. Fiedler, K. Müller, P. Nielsen, G. Thomas, N. Gengler, G. de Jong, C. Ødegard, F. Machioldi, F. Miglior, M. Alsaaaod and JB. Cole. 2018. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. TBC: 1-21 https://doi.org/10.3168/jds.2017-13531.
- ↑ Van der Spek, D., J.A.M. van Arendonk, A.A.A. Vallée, and H. Bovenhuis. 2013. Genetic parameters for claw disorders and the effect of preselecting cows for trimming. J. Dairy Sci. 96:6070–6078. doi:10.3168/jds.2013-6833.
- ↑ Heringstad, B., C. Egger-Danner, N. Charfeddine, J.E. Pryce, K.F. Stock, J. Kofler, A.M. Sogstad, M. Holzhauer, A. Fiedler, K. Müller, P. Nielsen, G. Thomas, N. Gengler, G. de Jong, C. Ødegard, F. Machioldi, F. Miglior, M. Alsaaaod and JB. Cole. 2018. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. TBC: 1-21 https://doi.org/10.3168/jds.2017-13531.
- ↑ Egger-Danner, C., P. Nielsen, A. Fiedler, A. Müller, T. Fjeldaas, D. Döpfer, V. Daniel, C. Bergsten, G. Cramer, A.-M. Christen, K.F. Stock, G. Thomas, M. Holzhauer, A. Steiner, J. Clarke, N. Capion, N. Charfeddine, J.E. Pryce, E. Oakes, J. Burgstaller, B. Heringstad, C. Ødegård, J. Kofler, F. Egger, and J.B. Cole. 2015. ICAR Claw Health Atlas. ICAR Technical Series. No. 18. International Committee for Animal Recording, Rome, Italy.
- ↑ Heringstad, B., C. Egger-Danner, N. Charfeddine, J.E. Pryce, K.F. Stock, J. Kofler, A.M. Sogstad, M. Holzhauer, A. Fiedler, K. Müller, P. Nielsen, G. Thomas, N. Gengler, G. de Jong, C. Ødegard, F. Machioldi, F. Miglior, M. Alsaaaod and JB. Cole. 2018. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. TBC: 1-21 https://doi.org/10.3168/jds.2017-13531.
- ↑ Clarkson MJ, WB Faull, JW Hughes (1996): Incidence and prevalence of lameness in dairy cattle. Vet Rec 138: 563-567.
- ↑ Bergsten, C. (2001). Laminitis: Causes, Risk Factors, and Prevention, Texas Animal Nutrition Council. http://www.txanc.org/docs/BovineLaminitis.pdf.
- ↑ Somers J., Frankena K., Noordhuizen-Stassen E., Metz J. 2005. Risk factors for digital dermatitis in dairy cows kept in cubicle houses in The Netherlands. Prev. Vet. Med. 71: 11–21.
- ↑ Holzhauer M., Hardenberg C., Bartels C., Frankena K. Herd- and cow-level prevalence of digital dermatitis in the Netherlands and associated factors. J. Dairy Sci. 2006; 89: 580–588.
- ↑ Cramer, G. 2018. Personal communication.
- ↑ Mülling C.K.W., L. Green, Z. Barker, J. Scaife, J. Amory, M. Speijers. 2005. Risk factors associated with foot lameness in dairy cattle and a suggested approach for lameness reduction. World Buiatrics Congress, Nice, France.
- ↑ Sprecher, D.J., D. E. Hostetler and J.B. Kaneene. 1997. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology, Vol. 47 (6):1179-1187.
- ↑ von Keyserlingk, M. A. G., J. Rushen, A. M. de Passillé, and D. M. Weary. 2009. Invited review: The welfare of dairy cattle-key concepts and the role of science. J. Dairy Sci. 92:4101–4111.
- ↑ Flower, F. C. and D. M. Weary. 2009. Gait assessment in dairy cattle. Animal 3:1, pp 87–95.
- ↑ Green, L. E., V. J. Hedges, Y. H. Schukken, R. W. Blowey, and A. J. Packington. 2002. The impact of clinical lameness on the milk yield of dairy cows. J. Dairy Sci. 85:2250–2256.
- ↑ Bicalho, R. C., V. S. Machado, and L. S. Caixeta. 2009. Lameness in dairy cattle: A debilitating disease or a disease of debilitated cattle? A cross-sectional study of lameness prevalence and thickness of the digital cushion. J. Dairy Sci. 92:3175–3184.
- ↑ Sanders, A. H., J. K. Shearer, and A. De Vries. 2009. Seasonal incidence of lameness and risk factors associated with thin soles, white line disease, ulcers, and sole punctures in dairy cattle. J. Dairy Sci. 92:3165-3174.
- ↑ DeFrain, J. M., M. T. Socha, and D. J. Tomlinson. 2013. Analysis of foot health records from 17 confinement dairies. J. Dairy Sci. 99: 7329-7339.
- ↑ Douglas M., L. Solano and K. Orsel. 2019. The surprising relationship between lameness and hoof lesions. Progressive Dairyman, 31st May.
- ↑ Solano, L., H. W. Barkema. E. A. Pajor, S. Mason, S. LeBlanc, J. C. Zaffino Heyerhoff, C. G. R. Nash, D. B. Haley, E. Vasseur, D. Pellerin, J. Rushen, A. M. de Passillé and K. Orsel. 2015. Prevalence of lameness and associated risk factors in Canadian Holstein-Friesian cows housed in free stall barns. J. Dairy Sci. 98:6978–6991.
- ↑ Beggs, D. S., E. C. Jongman, P. H. Hemsworth and A. D. Fisher. 2019. Lame cows on Australian dairy farms: A comparison of farmer-identified lameness and formal lameness scoring, and the position of lame cows within the milking order. J. Dairy Sci.102:1522–1529.
- ↑ Hund, A., Chiozza Logroño, J., Ollhoff, R.D., Kofler, J. 2019. Aspects of lameness in pasture based dairy systems. Vet. J. 244: 83–90.
- ↑ Randall L. V., M. J. Green, M. G. G. Chagunda, C. Mason, S. C. Archer, L. E. Green, and J. N. Huxley. 2015. Low body condition predisposes cattle to lameness: An 8-year study of one dairy herd. J. Dairy Sci. 98:3766–3777.
- ↑ Brenninkmeyer, C., S. Dippel, S. March, J. Brinkmann, C. Winckler and U. Knierim. 2007. Reliability of a subjective lameness scoring system for dairy cows. Animal Welfare 16:127–129.
- ↑ Flower, F. C. and D. M. Weary. 2009. Gait assessment in dairy cattle. Animal 3:1, pp 87–95.
- ↑ Sogstad Å. M., T. Fjeldaas and O. Østerås. 2012. Locomotion score and claw disorders in Norwegian dairy cows assessed by claw trimmers. Livestock Science, Vol. 144, p.157-162.
- ↑ Sprecher, D.J., D. E. Hostetler and J.B. Kaneene. 1997. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology, Vol. 47 (6):1179-1187.
- ↑ Schlageter-Telloa, A., E. A. M. Bokkers, P. W. G. Groot Koerkampa, T. Van Hertemd, S. Viazzid, C. E. B. Romaninid, I. Halachmie, C. Bahrd, D. Berckmansd, and K. Lokhorsta. 2014. Manual and automatic locomotion scoring systems in dairy cows: A review. Prev. Vet. Med. 116:12–25.
- ↑ 153.0 153.1 March, S., J. Brinkmann and C. Winkler. 2007. Effect of training on the inter-observer reliability of lameness scoring in dairy cattle. Anim. Welfare 16:131–133.
- ↑ Garcia, E., K. König, B.H. Allesen-Holm, C. Klaas, J.M. Amigo, R. Bro, and C. Enevoldsen. 2015. Experienced and inexperienced observers achieved relatively high within-observer agreement on video mobility scoring of dairy cows. J. Dairy Sci. 98:4560–4571.
- ↑ Beggs, D. S., E. C. Jongman, P. H. Hemsworth and A. D. Fisher. 2019. Lame cows on Australian dairy farms: A comparison of farmer-identified lameness and formal lameness scoring, and the position of lame cows within the milking order. J. Dairy Sci.102:1522–1529.
- ↑ Beggs, D. S., E. C. Jongman, P. H. Hemsworth and A. D. Fisher. 2019. Lame cows on Australian dairy farms: A comparison of farmer-identified lameness and formal lameness scoring, and the position of lame cows within the milking order. J. Dairy Sci.102:1522–1529.
- ↑ Telezhenko, E. and C. Bergsten. 2005. Influence of floor type on the locomotion of dairy cows. App. Ani. Beh. Sci. 93:183–197.
- ↑ Cook, N. 2005. A Guide to Investigating a Herd Lameness Problem. 17 p. University of Wisconsin-Madison, USA.
- ↑ Flower, F. C., A. M. de Passillé, D. M. Weary, D. J. Sanderson, and J. Rushen. 2007. Softer, higher-friction flooring improves gait of cows with and without sole ulcers. J. Dairy Sci. 90:1235–1242.
- ↑ 160.0 160.1 Groenevelt, M., D. C. J. Main, D. Tisdall, T. G. Knowles and N. J. Bell. 2014. Measuring the response to therapeutic foot trimming in dairy cow with fortnightly lameness scoring. Vet. J. 201:283-288.
- ↑ Eriksson, H. K., R. R. Daros, M. A. G. von Keyserlingk, and D. M. Weary. 2020. Effects of case definition and assessment frequency on lameness incidence estimates. J. Dairy Sci. 103 – Article in Press.
- ↑ Laursen, M. V., D. Boelling and T. Mark. 2009. Genetic parameters for claw and leg health, foot and leg conformation, and locomotion in Danish Holsteins. J. Dairy Sci. 92:1770-1777.
- ↑ Weber, A., E. Stamer, W. Junge, and G. Thaller. 2013. Genetic parameters for lameness and claw and leg diseases in dairy cows. J. Dairy Sci. 96:3310–3318.
- ↑ Egger-Danner, C., A. Koeck, J. Kofler, J. Burgstaller, F. Steininger, C. Fuerst, and B. Fürst-Waltl. 2017. Evaluation of different data sources for genetic improvement of claw health in Austrian Fleckvieh (Simmental) and Brown Swiss cattle. Page 294 in Proc. 19th Int. Symp. 11th Int. Conf. Lameness in Ruminants, Munich, Germany.
- ↑ Eriksson, H. K., R. R. Daros, M. A. G. von Keyserlingk, and D. M. Weary. 2020. Effects of case definition and assessment frequency on lameness incidence estimates. J. Dairy Sci. 103 – Article in Press.
- ↑ Haladjian, J., J. Haug, S. Nüske, and B. Bruegge. 2018. A wearable sensor system for lameness detection in dairy cattle. Multimodal Technol. Interact. 2:27.
- ↑ Kofler, J. 2015. Klauenerkrankungen in Österreich – Wirtschafliche Aspekte, Häufigkeiten, Erkennung & fütterungsbedingte ursachen. ZAR Seminar, Vienna, Austria.
- ↑ Ahlén L. and T. Fjeldaas. 2019. Digital dermatitis and lameness: An evaluation of locomotion scoring as a tool to detect and control the disease. Proc. 20th Int. Symp. and 12th Int. Conference on Lameness in Ruminants, Asakusa, Japan, p. 200.
- ↑ Flower, F. C., D. J. Sanderson and D. M. Weary. 2006. Effects of milking on dairy cow gait. J Dairy Sci. 89:2084-2089.
- ↑ Fjeldaas, T., Å. M. Sogstad and O. Østerås. 2011. Locomotion and claw disorders in Norwegian dairy cows housed in free stalls with slatted concrete, solid concrete, or solid rubber flooring in the alleys. J. Dairy Sci. 94:1243-1255.
- ↑ Kofler, J. 2013. Computerised claw trimming database programs – the basis for monitoring hoof health in dairy herds. Vet. J. 198: 358–361.
- ↑ Green, L. E., V. J. Hedges, Y. H. Schukken, R. W. Blowey, and A. J. Packington. 2002. The impact of clinical lameness on the milk yield of dairy cows. J. Dairy Sci. 85:2250–2256.
- ↑ Egger-Danner, C., A. Koeck, J. Kofler, J. Burgstaller, F. Steininger, C. Fuerst, and B. Fürst-Waltl. 2017. Evaluation of different data sources for genetic improvement of claw health in Austrian Fleckvieh (Simmental) and Brown Swiss cattle. Page 294 in Proc. 19th Int. Symp. 11th Int. Conf. Lameness in Ruminants, Munich, Germany.
- ↑ Randall L. V., M. J. Green, L. E. Green, M. G. G. Chagunda, C. Mason, S. C. Archer, and J. N. Huxley. 2018. The contribution of previous lameness events and body condition score to the occurrence of lameness in dairy herds: A study of 2 herds. J. Dairy Sci. 101:1311–1324.
- ↑ Whay, H. R., A. E. Waterman and A. J. F. Webster. 1997. Associations between locomotion, claw lesions and nociceptive threshold in dairy heifers during the peri-partum period. Vet. J. 154:155-161.
- ↑ Heringstad, B. C. Egger-Danner, N. Charfeddine, J.E. Pryce, K.F. Stock, J. Kofler, A.M. Sogstad, M. Holzhauer, A. Fiedler, K. Müller, P. Nielsen, G. Thomas, N. Gengler, G. de Jong, C. Ødegård, F. Malchiodi, F. Miglior, M. Alsaaod, and J. B. Cole. 2018. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. 101:4801–4821.
- ↑ Whay, H. R., D. C. J. Main, L. E. Green and A. J. F. Webster. 2003. Assessment of the welfare of dairy cattle using animal-based measurements: direct observations and investigation of farm records. Vet. R. 153:197-202.
- ↑ Leach, K. A., H. R. Whay, C. M. Maggs, Z. E. Barker, E. S. Paul, A. K. Bell and D. C. J. Main. 2010. Working towards a reduction in cattle lameness: 2. Understanding dairy farmers’ motivations. Res. Vet. Sci. 89:318-323.
- ↑ Leach, K. A., D. A. Tisdall, N. J. Bell, D. C. J. Main and L. E. Green. 2010. The effects of early treatment for hind limb lameness in dairy cows on four commercial UK farms. Vet. J. 193:626-632.
- ↑ Ring, S. C., A. J. Twomey, N. Byrne, M. M. Kelleher, T. Pabiou, M. L. Doherty, and D. P. Berry. 2018. Genetic selection for hoof health traits and cow mobility scores can accelerate the rate of genetic gain in producer scored lameness in dairy cows. J. Dairy Sci. 101:10034–10047.
- ↑ Egger-Danner, C., A. Koeck, J. Kofler, J. Burgstaller, F. Steininger, C. Fuerst, and B. Fürst-Waltl. 2017. Evaluation of different data sources for genetic improvement of claw health in Austrian Fleckvieh (Simmental) and Brown Swiss cattle. Page 294 in Proc. 19th Int. Symp. 11th Int. Conf. Lameness in Ruminants, Munich, Germany.
- ↑ Berry, S. L., D. H. Read, R. L. Walker, and T. R. Famula. 2010. Clinical, histologic, and bacteriologic findings in dairy cows with digital dermatitis (footwarts) one month after topical treatment with lincomycin hydrochloride or oxytetracycline hydrochloride. J. Am. Vet. Med. Assoc. 237:555–560.
- ↑ Parker Gaddis, K. L., J. B. Cole, J. S. Clay, and C. Maltecca. 2014. Genomic selection for producer-recorded health event data in US dairy cattle. J. Dairy Sci. 97:3190–3199.
- ↑ Koeck, A., B. Fuerst-Waltl, F. Steininger, and C. Egger-Danner. 2016. Genetic parameters for body weight, body condition score and lameness in Austrian dairy cows. Interbull Bull. 50:51–53.
- ↑ Koeck, A., M. Ledinek, L. Gruber, F. Steininger, B. Fuerst-Waltl, and C. Egger-Danner. 2018. Genetic analysis of efficiency traits in Austrian dairy cattle and their relationships with body condition score and lameness. J. Dairy Sci. 101:445-455.
- ↑ Sprecher, D.J., D. E. Hostetler and J.B. Kaneene. 1997. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology, Vol. 47 (6):1179-1187.
- ↑ Flower, F. C., D. J. Sanderson and D. M. Weary. 2006. Effects of milking on dairy cow gait. J Dairy Sci. 89:2084-2089.
- ↑ Koeck, A., B. Fuerst-Waltl, F. Steininger, and C. Egger-Danner. 2016. Genetic parameters for body weight, body condition score and lameness in Austrian dairy cows. Interbull Bull. 50:51–53.
- ↑ Egger-Danner, C., A. Koeck, J. Kofler, J. Burgstaller, F. Steininger, C. Fuerst, and B. Fürst-Waltl. 2017. Evaluation of different data sources for genetic improvement of claw health in Austrian Fleckvieh (Simmental) and Brown Swiss cattle. Page 294 in Proc. 19th Int. Symp. 11th Int. Conf. Lameness in Ruminants, Munich, Germany.
- ↑ Rouha-Mülleder, C., C. Iben, E. Wagner, G. Laaha, J. Troxler, and S. Waiblinger. 2009. Relative importance of factors influencing the prevalence of lameness in Austrian cubicle loose-housed dairy cows. Prev. Vet. Med. 92:123–133.
- ↑ Weber, A., E. Stamer, W. Junge, and G. Thaller. 2013. Genetic parameters for lameness and claw and leg diseases in dairy cows. J. Dairy Sci. 96:3310–3318.
- ↑ Heringstad, B. C. Egger-Danner, N. Charfeddine, J.E. Pryce, K.F. Stock, J. Kofler, A.M. Sogstad, M. Holzhauer, A. Fiedler, K. Müller, P. Nielsen, G. Thomas, N. Gengler, G. de Jong, C. Ødegård, F. Malchiodi, F. Miglior, M. Alsaaod, and J. B. Cole. 2018. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. 101:4801–4821.
- ↑ Berry, D.P., M.L. Bermingham, M. Godd and S.J. More. 2011. Genetics of animal health and disease in cattle. I. Vet. J. 64:5.
- ↑ Heringstad, B. C. Egger-Danner, N. Charfeddine, J.E. Pryce, K.F. Stock, J. Kofler, A.M. Sogstad, M. Holzhauer, A. Fiedler, K. Müller, P. Nielsen, G. Thomas, N. Gengler, G. de Jong, C. Ødegård, F. Malchiodi, F. Miglior, M. Alsaaod, and J. B. Cole. 2018. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. 101:4801–4821.
- ↑ Ring, S. C., A. J. Twomey, N. Byrne, M. M. Kelleher, T. Pabiou, M. L. Doherty, and D. P. Berry. 2018. Genetic selection for hoof health traits and cow mobility scores can accelerate the rate of genetic gain in producer scored lameness in dairy cows. J. Dairy Sci. 101:10034–10047.
- ↑ Heringstad, B. C. Egger-Danner, N. Charfeddine, J.E. Pryce, K.F. Stock, J. Kofler, A.M. Sogstad, M. Holzhauer, A. Fiedler, K. Müller, P. Nielsen, G. Thomas, N. Gengler, G. de Jong, C. Ødegård, F. Malchiodi, F. Miglior, M. Alsaaod, and J. B. Cole. 2018. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. 101:4801–4821.
- ↑ Koeck, A., B. Fuerst-Waltl, J. Kofler, J. Burgstaller, F. Steininger, C. Fuerst, and C. Egger-Danner. 2019. Short communication: Use of lameness scoring to genetically improve claw health in Austrian Fleckvieh, Brown Swiss, and Holstein cattle. J. Dairy Sci. 102:1397–1401.
- ↑ Garcia, E., K. König, B.H. Allesen-Holm, C. Klaas, J.M. Amigo, R. Bro, and C. Enevoldsen. 2015. Experienced and inexperienced observers achieved relatively high within-observer agreement on video mobility scoring of dairy cows. J. Dairy Sci. 98:4560–4571.
- ↑ Winckler, C. and S. Willen. 2001. The reliability and repeatability of a lameness scoring system for use as an indicator of welfare in dairy cattle. Acta Agric. Scand. Anim. Sci. Suppl. 30:103–107.
- ↑ Leach, K. A., H. R. Whay, C. M. Maggs, Z. E. Barker, E. S. Paul, A. K. Bell and D. C. J. Main. 2010. Working towards a reduction in cattle lameness: 2. Understanding dairy farmers’ motivations. Res. Vet. Sci. 89:318-323.
- ↑ 201.0 201.1 Gibbons, J., D. B. Haley, J. Higginson Cutler, C. Nash, J. Zaffino, D. Pellerin, S. Adam, A. Fournier, A. M. de Passillé, J. Rushen and E. Vasseur. 2014. Technical note: A comparison of 2 methods of assessing lameness prevalence in tiestall herds. J. Dairy Sci. 97:350-353.
- ↑ Alsaaod, M., C. Syring, M. Luternauer, M. G. Doherr, and A. Steiner. 2015. Effect of routine claw trimming on claw temperature in dairy cows measured by infrared thermography. J. Dairy Sci. 98:2381–2388. doi:10.3168/jds.2014-8594
- ↑ Beer, G., M. Alsaaod, A. Starke, G. Schuepbach-Regula, H. Müller, P. Kohler, P. and A. Steiner. 2016. Use of extended characteristics of locomotion and feeding behavior for automated identification of lame dairy cows, PLOS ONE 11:e0155796.
- ↑ 204.0 204.1 Nechanitzky, K., A. Starke, B. Vidondo, H. Müller and M. Reckardt. 2016. Analysis of behavioral changes in dairy cows associated with claw horn lesions. J. Dairy Sci. 99:2904–2914. doi:10.3168/jds.2015-10109.
- ↑ Barker, Z. E., J. R. Amory, J. L. Wright, S. A. Mason, R. W. Blowey and L. E. Green. 2009. Risk factors for increased rates of sole ulcers, white line disease, and digital dermatitis in dairy cattle from twenty-seven farms in England and Wales. J. Dairy Sci. 92: 1971–1978. doi:10.3168/jds.2008-1590.
- ↑ de Mol, R. M., A. G., Bleumer, E. J. B., J. T. N. van der Werf, and Y. de Haas. 2013. Applicability of day-to-day variation in behavior for the automated detection of lameness in dairy cows, J. Dairy Sci. 96:3703–3712.
- ↑ 207.0 207.1 Beer, G., M. Alsaaod, A. Starke, G. Schuepbach-Regula, H. Müller, P. Kohler, P. and A. Steiner. 2016. Use of extended characteristics of locomotion and feeding behavior for automated identification of lame dairy cows, PLOS ONE 11:e0155796.
- ↑ Giuliana, G. M.-P., J. Kaler, J. Remnant, L. Cheyne, and C. Abbott. 2014. Behavioural changes in dairy cows with lameness in an automatic milking system, Applied Ani. Behavioural Science 150: 1-8.
- ↑ Alsaaod, M. and W. Buscher. 2012. Detection of hoof lesions using digital infrared thermography in dairy cows, J. Dairy Sci. 95: 735–742.
- ↑ Stokes, J.E., K. A. Leach, D. C. Main, and H. R. Whay. 2012. An investigation into the use of infrared thermography (IRT) as a rapid diagnostic tool for foot lesions in dairy cattle, Vet. J. 193: 674–678.
- ↑ Alsaaod, M., C. Syring, J., Dietrich, M. G. Doherr, T. Gujan and A. Steiner. 2014. A field trial of infrared thermography as a non-invasive diagnostic tool for early detection of digital dermatitis in dairy cows, Vet. J. 199:281–285.
- ↑ Wilhelm, K., J. Wilhelm, and M. Furll. 2015. Use of thermography to monitor sole haemorrhages and temperature distribution over the claws of dairy cattle. Vet. Rec. 176: 146. doi:10.1136/vr.101547.
- ↑ Cook, N. 2005. A Guide to Investigating a Herd Lameness Problem. 17 p. University of Wisconsin-Madison, USA.
- ↑ Heringstad, B., Y.M. Chang, M. Svendsen, and D. Gianola. 2007. Genetic analysis of calving difficulty and stillbirth in Norwegian Red cows. Journal of Dairy Science 90: 3500-3507. https://doi.org/10.3168/jds.2006-792
- ↑ Cole, J.B., G.R. Wiggans, and P.M. VanRaden. 2007. Genetic evaluation of stillbirth in United States Holsteins using a sire-maternal grandsire threshold model. J Dairy Sci. 90:2480-2488. https://doi.org/10.3168/jds.2006-435
- ↑ Eaglen, S.A., M.P. Coffey, J.A. Woolliams, and E. Wall. 2012. Evaluating alternate models to estimate genetic parameters of calving traits in United Kingdom Holstein-Friesian dairy cattle. Genet. Sel. Evol. 44(1):23. doi: 10.1186/1297-9686-44-23
- ↑ Snell, E. J. 1964. A Scaling Procedure for Ordered Categorical Data. Biometrics Vol. 20, No. 3 (Sep., 1964), pp. 592-607. https://doi.org/10.2307/2528498
- ↑ Heringstad, B., Y.M. Chang, M. Svendsen, and D. Gianola. 2007. Genetic analysis of calving difficulty and stillbirth in Norwegian Red cows. Journal of Dairy Science 90: 3500-3507. https://doi.org/10.3168/jds.2006-792
- ↑ GENEX. 2022. How much calving ease is enough? Available at https://genex.coop/how-much-calving-ease-is-enough/
- ↑ Haskell (2019). Mapping the global use of welfare indicators for dairy cows.https://www.icar.org/Documents/Prague-2019/Presentations/02%20-%20Marie%20Haskell.pdf
- ↑ OIE. 2020: Terrestrial Animal Health Code. https://rr-europe.oie.int/wp-content/uploads/2020/08/oie-terrestrial-code-1_2019_en.pdf.












