Guidelines Section 24: Recording resilience in sheep and goats
Guidelines on recording resilience traits and the environment in sheep and goats
Change Summary
| Date of change | Nature of Change |
| October 2024 | First version |
Introduction and scope
Introduction
The present guidelines aim at addressing resilience traits in small ruminants, as well as the description of the environment.
These recommendations are mainly based on a work achieved in the SMARTER H2020 project (n° 772787) whose objective was to promote harmonisation and international cooperation on breeding processes in small ruminant, especially those concerning the selection of efficiency and resilience. In this project, case studies of across country genetic evaluation, implemented as a proof of concept, have highlighted the importance of analysing traits that have been collected and/or calculated on a same way across country. Therefore, it appears fundamental that novel traits, such as resilience-related traits, which are not still widely routinely recorded on-farm for selection purposes, be recorded identically, or at least in the most similar way as possible. For that purpose, recommendations must be proposed, for countries or breeding organisations that would like to start to record efficiency or resilience traits, or that would like to set up an across-country genetic evaluation on these traits. The more similar the traits, the higher the genetic correlation across country (at same level of connection across country).
In addition, as resilience may be considered as basically related to the environmental challenges such as nutritional, disease or climatic challenges, the documentation of the environment is also described. Tackling the record of the environment is a novelty in selection of small ruminant.
The recommendations issued in a deliverable of the SMARTER project have been basically written by the partners of the project working on tasks dedicated to the different resilience-related traits and as well by the Sheep, Goat and Camelid ICAR Working Group. The Working Group was indeed involved, as partner for some of the members, as stakeholders for some other, and through ICAR who was a partner itself. Therefore, these guidelines are the fruit of a close cooperation between many academic and non-academic co-authors. Materials were also collected from results obtained in other projects (e.g. H2020 iSAGE, POCTEFA ARDI).
The recommendations, even though they target to suggest people measuring and calculating the traits the same way, are more informative than normative. The different ways to measure and calculate the traits are presented, without imposing one way, yet while suggesting some general features. Five sub-sections of recommendations were written: health and disease, survival of foetus and young, behaviour, lifetime resilience, record of the environment. All sub-sections are written with the same template and are consistent by themselves.
All the recommendations are based on the current state of the art. However, they are meant to evolve with new results and new research, and they are meant to be enhanced, consolidated, enriched. It is possible to add a new trait, a new proxy, a new sub-section. In brief, the recommendations must keep alive to stick to the evolving state of the art. This implies that the consortium that produced these recommendations, in some way, continue to contribute. ICAR, with its working group dedicated to sheep and goat, is the relevant organisation to collect and integrate the different novelties and contributions.
Scope
The SMARTER recommendations cover the following fields, shown in the figure 1.
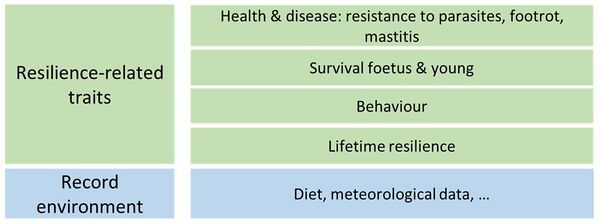
The resilience-related traits are: health and disease (with a focus on resistance to parasites, to footrot, and to mastitis), survival foetus and young, behaviour traits (with a focus on behavioural reactivity towards conspecifics or humans, maternal reactivity, behaviour at grazing), lifetime resilience.
The record of the environment covers the meteorological data and the diet. The record of the rations was studied in the on-farm protocols of SMARTER-WP1, especially in France. The record of the meteorological data benefited from works carried out in the H2020 iSAGE and POCTEFA ARDI projects, some of the SMARTER partners being committed in those projects.
The recommendations are conceived to be evolutive. Amendments can be brought in the next years, especially when the recommendations will turn into ICAR guidelines, either to strengthen results or include new insights, or to add new sub-sections or new traits. For example: (i) in the record of the environment, sensor data may be included; (ii) new disease whose resistance has a genetic component.
Definition of resilience
In these guidelines, we use the following definition of the resilience.
Resilience is defined as the ability of an animal/system to either maintain or revert quickly to high production and health status when exposed to a diversity of challenges, with a focus on nutritional and/or health challenges. Resilience is therefore the trajectory that captures the deviation from, and recovery to, the unchallenged state. Direct indicators of health and welfare will address gastro-intestinal parasitism, lameness (footrot) and mastitis, the most economically important endemic diseases of small ruminants. Indirect indicators of health and welfare of economic importance for breeders are lamb and foetal survival, functional longevity, maternal and lamb behaviour, and neonatal vigour..
Recording of resilience
This section lists the different groups of resilience traits addressed by the ICAR guidelines. For more clarity, each group of traits is then described in a specific document that has its own template and is consistent by itself. The list of (groups of) traits may be updated. The description of each group of traits may be updated as well when needed.
Health and disease: recording the resistance to parasites, to footrot and to mastitis in sheep and goat
Change summary
| Date of change | Nature of Change |
| October 2024 | First version |
| December 2024 | Tracked change revisions by MS |
Introduction and scope
Introduction
Livestock diseases cause significant economic losses due reduced productivity, failing to express the genetic potential of animals, treatment costs, and consequently the culling of animals. Therefore, health and resistance to disease are keys factors for increasing resilience in farm animals in general and in small ruminants in particular. Among the challenges that sheep and goats must face, the infectious challenges are among the most important. They lead to losses of production and difficulties of reproduction. They also generate an increase in the consumption of chemical input. Beyond actual extra cost that may hamper the sustainability of the farms, but also of the breeding programs, there is a risk for the environment and the occurrence of resistance to drugs.
In most cases, an integrated approach is the more beneficial and efficient, mixing the different leverages. Among them, the control of the challenges by the host through its genetic resistance has shown its efficiency for some disease (resistance to scrapie, resistance to mastitis in dairy species) or is promising (resistance to parasites, resistance to footrot).
These guidelines on health and disease phenotypes are dedicated to any kind of health and disease resistance indicators. However, to start, we focus on the traits studied in SMARTER, which are the resistance to parasites and the resistance to footrot and mastitis in meat sheep and dairy sheep and goats.
Scope
This section on recording health and disease in sheep and goats starts following the task achieved in SMARTER and includes the following three sub-sections:
- Resistance to parasites
- Resistance to mastitis
- Resistance to footrot
Resistance to parasites
Definition, terminology, rationale
Resistance may be defined as the host’s ability to limit its parasite load (Råberg et al., 2007). The resistance to parasites described here corresponds to the resistance to gastro-intestinal nematodes (GIN). They are one of the main constraints for grazing sheep. They cause substantial economic losses due to lower production levels, the costs of anthelmintic treatments and the mortality of severely affected sheep. GIN control strategies mainly rely on treatment with anthelmintics. In many regions of the world, studies have reported the development of GIN resistance to most anthelmintic molecules due to their extensive use. Additionally, the possible presence of drug residues in animal products and the negative impact of these molecules on the micro and macro fauna of the soil are of concern. Therefore, sustainable GIN control may be a priority with schemes that do not only rely on anthelmintics but include complementary strategies such as nutritional supplementation with tannins and/or proteins, pasture management, and genetic selection of resistant animals. This latter strategy relies on the existence of genetic variation of host resistance to GIN both between and within breeds.
The faecal egg count (FEC), which is the number of parasite eggs per gram of faeces, is the most commonly used indicator to assess this resistance to GIN. In many countries, the selection for parasite resistance is based on FEC measures in natural infestation conditions under natural grazing conditions. As FEC measurements in sheep and goats are extremely costly and laborious, and because response to artificial challenges is highly correlated to response to natural infestation, it is therefore possible to implement a protocol of experimental infestation, as it is the case in France.
Beside FEC, different phenotypes can be used to measure resistance to GINs such as packed cell volume (PCV), FAffa MAlan CHArt (FAMACHA©) score, DAG score, immunological traits, and blood bepsinogen dosing (Shaw et al., 2012; Bishop, 2012; Bell et al., 2019; Sabatini et al., 2023).
Data recording
Indicators of parasite resistance or resilience
Faecal Egg Count
Faecal Egg Count (FEC) is the main indicator that measures the egg excretion intensity. It measures the number of parasite eggs per gram of faeces. This trait is related to the resistance of the animal (ability to limit the installation, the development and the prolificacy of the nematode inside the digestive tract (especially the abomasum). FEC is determined for each sample using a modified MacMaster technique (Whitlock, 1948 or Raynaud, 1970) with a sensitivity of 100 or 15 eggs per gram, respectively. The measure may be done in natural or in experimental infestation. FEC can be applied to one species (for example Haemonchus contortus (Hc)) or several species (including Hc, Teladorsagia circumcincta, Trichostrongylus colubriformis, etc).
The distribution of the FEC has an asymmetric distribution (some high value, many low or medium value). A transformation must be applied to process a genetic analysis. The most frequent transformations are a root (fourth, third or square root) or a log transformation.
Packed Cell Volume
Packed Cell Volume (PCV) - Blood samples were collected in EDTA coated tubes and PCV values were determined individually by centrifugation in microhematocrit tubes with a relative centrifugal force of 9500 for 10 min.
PCV can be exploited as a single value of more relevantly as a gain/loss of PCV between two points. Variation of PCV is a relevant indicator of the resilience of the sheep / goat.
FAMACHA score
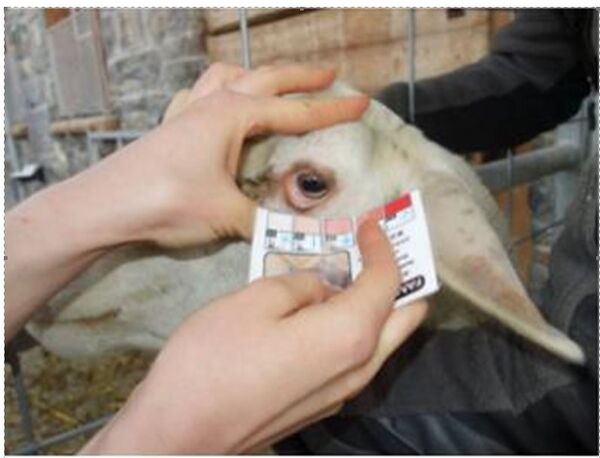
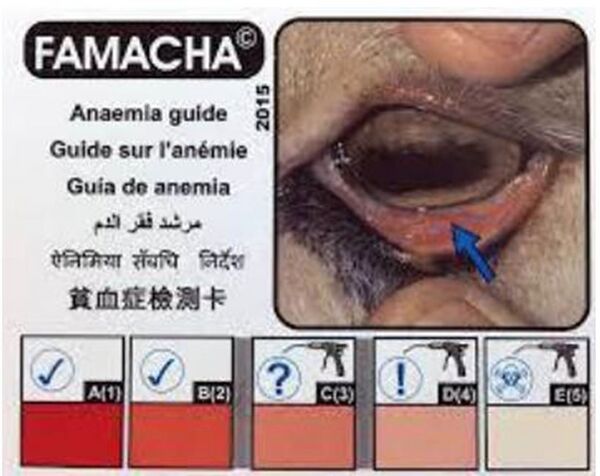
FAMACHA® score – As the anaemia provoked by some hematophagous parasites is at some stage visible on the mucosa (especially ocular mucosa), a scale of grading, based on the colour of the ocular mucosa, ranging from 1 (dark red mucosa) to 5 (white mucosa) has been built. This score was developed in South Africa to facilitate the clinical identification of anaemic sheep infected with H. contortus (Van Wyk and Bath, 2002).
As drawbacks, the FAMACHA® score does not allow to detect the non-hematophagous parasites and it appears quite belatedly: a FAMACHA® score over 3 concerns animals with a PCV below 20%. The method is not specific, anaemia being possibly caused by other reason than Haemonchus contortus. It is however interesting to detect the anaemia. FAMACHA® score is related to the resilience of the sheep / goat.
DAG score
DAG score is an indicator for assessing dagginess, which measures faecal soiling in sheep. DAG score uses a 5-point or 6-point scoring scale ranging from 0 (no dags) to 5 or 6 (very daggy). Dag score scale shows the degree or extent of faecal contamination of the fleece.
The key is to be consistent when scoring a mob of sheep and for these sheep to have been run under similar conditions. Faecal contamination should not be confused with urine stain in ewe lambs and hoggets.
Immunological traits
Immunological and physiological profiles may be linked to phenotypes of resistance to parasites (strongyles). These new immunological and physiological profiles are blood lymphocytes cytokine production and serum levels of nematode parasite-specific Immunoglobulin A (IgA) that are produced upon whole blood stimulation. In SMARTER experiment in SRUC, blood was stimulated with pokeweed mitogen (a lectin that non-specifically activates lymphocytes irrespectively of their antigen specificity), and Teladorsagia circumcincta (T-ci) larval antigen to activate parasite-specific T lymphocytes.
Adaptive immune response may be determined by quantifying:
- cytokines interferon-gamma (IFN-γ), which relate to T-helper type 1 (Th1),
- interleukin IL-4, which relates to T-helper type 2 (Th2) and
- interleukin IL-10, which relate to regulatory T cell (Treg) responses.
Each immune trait displays a significant genetic variation (heritabilities ranging from 0.14 to 0.77). Heritability of IgA is moderate (0.41). Correlations with FEC are rather weak, from 0 to 0.27 but not significantly different from 0.
Blood Pepsinogen dosing
Blood pepsinogen is an indicator of the integrity of the gastric mucosa. The determination of serum pepsinogen is therefore a proxy in the diagnosis of abomasal strongylosis of ruminants (pepsinogen in blood is caused by an increase in the permeability of the abomasum mucosa due to presence of nematodes). There is a correlation between the concentration of pepsinogen in the blood and the number of worms harboured by the host.
Natural infestation
General considerations
Measurements (FEC or other proxies) are mainly undertaken in natural infestation under natural grazing conditions. In natural condition of infestation, frequency and amounts of yearly samplings have to be assessed according to the climate and epidemiological conditions and breeds. Local knowledge is essential for adjusting protocols to each country, as the level of infestation is strongly influenced by seasonality and the grazing system.
Several countries (e.g. Australia, New Zealand, and Uruguay), have incorporated the genetic evaluation of FEC at various ages into their national evaluation systems. In any case, in order to have data useful for the genetic evaluation, a representative sample of sheep in the flock involved in the selection scheme has to be periodically monitored to decide whether to sample the whole flock, i.e. when the number of infected animals and the level of infestation are considered sufficient to appreciate individual variability, individual FEC can be measured on the whole flock.
Further data related to environmental factors affecting the level of infestation should be recorded to be included in the genetic model for estimating the breeding values:
- Farm management mainly grazing system
- Birth type
- Sex
- Age of dam
- Parity
- Lambing date
- Sampling date
- Frequency, date, and molecule of anthelmintic administration
Additionally, stool cultures can be performed from the faecal samples taken (one per management group).
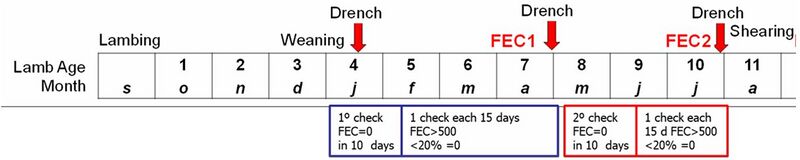
Description of the protocol and the measures (Uruguayan protocol)
At weaning, lambs undergo anthelmintic treatment, and their treatment efficacy is checked 8-14 days later through the analysis of FEC samples from 20 randomly selected lambs to confirm the absence of egg excretion. Subsequently, FEC is monitored every 15 days by collecting samples (based on epidemiological conditions) from 10-15% of lambs in each management group. The first individual FEC sampling is conducted when the FEC arithmetic mean exceeds 500 with no more than 20% samples exhibiting zero FEC. At this point, the lambs undergo anthelmintic treatment again, and their treatment efficacy is evaluated after 8-14 days. If the FEC mean remains above 500, a second individual sampling is conducted. Throughout the protocol, faecal egg counts (FEC1 and FEC2) are measured at the end of the first and second natural infestations. Generally, with some variations based on the breed, these samplings correspond to lambs at 9 and 11 months of age, respectively.
Currently, to simplify the protocol, only one sampling is conducted, and the control begins on a fixed date (early autumn) when the most significant parasite, H. contortus, prevails. Along with the FEC records (FEC1 and FEC2), other records, such as body weight, FAMACHA®, and body condition score, can also be taken.
Experimental infestation (French protocol)
As mentioned above, FEC measurements on sheep in commercial flocks are extremely costly and laborious. It has been shown that sheep that are selected on the basis of their response to artificial challenges respond similarly when exposed to natural infestation, and a high positive genetic correlation was estimated between FEC recorded under artificial or natural infestation. Moreover, it has been shown that selection of rams for parasite resistance after artificial challenges allows to improve the resistance of their female offspring for parasite infestation in natural conditions. Thus, an alternative approach may be to select rams gathered for AI progeny-testing or performance-testing by artificially infecting them with standardized doses of larvae.
In most cases, resistance to GIN is assessed in natural infestation conditions at grazing. However, the intensity of natural infestation in grazing animals depends on climatic conditions and may vary from season to season leading to over- or under-estimation of the genetic parameters of resistance. In France, sheep breeds are selected collectively on breeding stations and the strategy is to take advantage of this organization to implement the GIN control selection by phenotyping rams after experimental infestation. There are two main advantages. Firstly, a large diffusion of the genetic progress is expected via these rams, which are the future elite males. Secondly, the experimental infestation performed in control stations allow to evaluate these rams in homogeneous conditions (standardization of doses of infestation, farming conditions, climatic conditions, etc) during the ram evaluation period. Previous studies (Gruner et al., 2004) estimated high genetic correlations between resistances to experimental and natural infestation, between infestation by different parasite species (Haemonchus contortus and Trichostrongylus colubriformis) and between resistance in adult sheep and lambs. Moreover, recent works have shown that the genetic correlation between the resistance of rams in experimental conditions and the resistance of pregnant or milking ewes in natural conditions of GIN infestation are high.
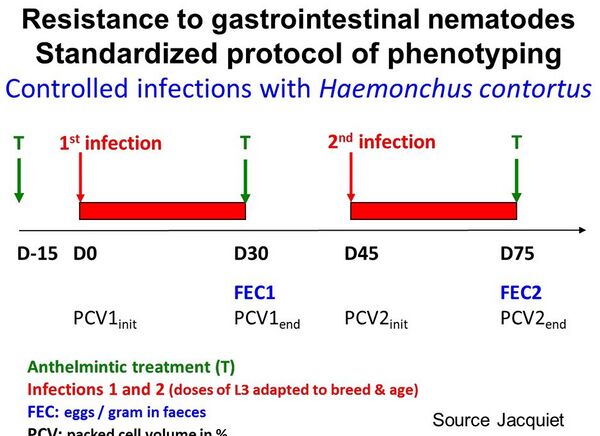
Description of the protocol and the measures
An original protocol for phenotyping resistance to gastro-intestinal parasitism has been conceived and developed in France, targeted to rams (or bucks) gathered in a breeding centre or station, or an AI centre (Jacquiet et al., 2015; Aguerre et al., 2018). Males bred indoors, supposed to be naïve, are artificially infected twice with L3 larvae of a given strain of Haemonchus contortus susceptible to anthelminthic. Males are subjected to a first infestation (after a coprological examination be performed to confirm that no eggs were excreted before the artificial infestation) with a given dose of L3 larvae (D0). At D30, the males are phenotyped (FEC30 and possibly PCV30) and treated with an anthelminthic. After a 15-day recovery period, the rams are challenged again with a given dose of L3 larvae of Haemonchus contortus. At that time (D45), the efficacy of anthelmintic treatment is ensured in each male. Thirty days after (D75) the second challenge, the males are phenotyped (FEC30 and possibly PCV30) and treated again. The protocol lasts 2 and a half months. During the protocol, the measures carried out are as follows:
- faecal egg counts (FEC30 and FEC75) at the end of the first and second infestation (from faecal sample).
- packed cell volumes PCV0, PCV30, PCV45 and PCV75 at the start and the end of both infestation (from blood sample).
Calculation of variables
The FEC30 and FEC75 are used per se. Variations of PCV are calculated:
- PCV_loss_inf1 = PCV0-PCV30 (or ratio PCV30/PCV0)
- PCV_loss_inf2 = PCV45-PCV75 (or ratio PCV75/PCV45)
- PCV_recovery = PCV45-PCV0
where PCV_loss_inf1 and PCV_loss_inf2 represent the loss of PCV after each infestation, while PCV_recovery represents the males’ capacity to recover after the first infestation.
PCV variations might be interpreted as an indicator of resilience of the animal, i.e. its ability to maintain its blood parameters despite the parasitical challenge.
Use for genetic analysis / genetic evaluation
Model for genetic analysis
The genetic analysis of experimentally infected animals that are raised indoors may include:
- Fixed effects: contemporary group (mob x doses of larvae), age of animals (eg. 1 year, 2 years, 3years, 4 years and older)
- Random additive effect of the animals
- Residual effect
The genetic analysis of naturally infected animals that are raised outdoors may include:
- Fixed effects: they obviously will depend of the type of animals (females in lactation vs lambs/kids). They should include flock/herd, year x season (e.g. spring, summer, autumn, winter), anthelmintic treatments (e.g. eprinomectin, ivermectin, moxidectin …) in interaction with the number of days between the date of treatment and the sampling date (e.g. less than 70 days, between 70 and 100 days, more than 100 days). For females in lactation: age and/or parity, litter size before lactation (single or multiple new-born lambs). For lambs or kids: age of the dam, type of birth or rearing, and age at the time of the records, expressed in day.
- Random additive effect of the animals
- Random permanent environment effect if repeated measures (e.g. for FEC 1 & 2)
- Residual effect
Genetic parameters
Pooled estimates of heritability to resistance to gastrointestinal parasites gained by meta-analysis (Mucha et al., 2022) in dairy goats and sheep and meat sheep are shown in Tables 1 and 2, while Table 3 shows the heritabilities estimated for the experimentally infected rams. In addition, we mention a paper from Casu et al (2022) in which a heritability of 0.21 for FEC was found in a 20 year follow-up study in an experimental flock in Sardinia, Italy.
Table 1. Pooled estimates of heritability of resistance to gastrointestinal parasites from meta- analysis in dairy goats and sheep.
| Trait1 | Species | Pooled h2(±SE) | Min2 h2 | Max3 h2 | N obs | N studies |
| FEC | Goats | 0.07 ± 0.01 | 0.04 | 0.15 | 8 | 2 |
| Sheep | 0.14 ± 0.04 | 0.09 | 0.35 | 6 | 3 |
1Trait: FEC – faecal egg count
2Minimum h2 from individual studies included in meta-analysis
3Maximum h2 from individual studies included in meta-analysis
Table 2. Pooled estimates of heritability of resistance to gastrointestinal parasites from meta- analysis in meat sheep (Mucha et al., 2022).
| Trait1 | Pooled h2 (±SE) | Min2 h2 | Max3 h2 | N obs | N studies |
| DAG | 0.30±0.06 | 0.06 | 0.63 | 37 | 15 |
| FCons | 0.14±0.02 | 0.03 | 0.27 | 13 | 5 |
| NBW4 | 0.10±0.02 | 0.00 | 0.54 | 11 | 3 |
| Par-Ab | 0.18±0.07 | 0.05 | 0.29 | 6 | 3 |
| Par-Ig | 0.36±0.06 | 0.13 | 0.67 | 24 | 8 |
| FEC | 0.29±0.03 | 0.00 | 0.82 | 118 | 32 |
| HC | 0.32±0.14 | 0.08 | 0.56 | 5 | 2 |
1Trait: DAG – dagginess, FCons – faecal consistency, NBW – number of worms, Par-Ab – parasitism anitbodies, Par-Ig – parasitism immunoglobulin, FEC –faecal egg count, HC - Haematocrit
2Minimum h2 from individual studies included in meta-analysis
3Maximum h2 from individual studies included in meta-analysis
4Pooled heritability obtained from a simple random effects model as the three level meta-analysis model did not converge
Table 3. Estimates of heritability of resistance to gastrointestinal parasites from meta-analysis in dairy sheep in experimental infestations (Aguerre et al., 2018)
| Trait1 | h2 |
| Root FEC_inf1 | 0.14±0.04 |
| RootFEC_inf2 | 0.35±0.08 |
| PCV_loss_inf1 | 0.24±0.05 |
| PCV_loss_inf2 | 0.18±0.06 |
| PCV-recovery | 0.16±0.06 |
Resistance to mastitis
Definition, terminology, rationale
In small ruminants, mastitis mainly consists in subclinical infections caused by coagulase- negative staphylococci, which is much more frequent than clinical mastitis (Bergonier et al., 2003). Under these conditions, somatic cell count (SCC) is an accurate, indirect measure to predict mammary gland infection. Therefore, SCC could be used as an indirect selection criterion for mastitis resistance as is widely done in dairy cattle. Moreover, selection for mastitis resistance in dairy sheep and goats could mainly focus on selection against subclinical mastitis using SCC, considering the low incidence of clinical cases in these species (<5%), compared to dairy cattle for which clinical cases occur frequently (Bergonier et al., 2003).
Clinical mastitis is not recorded in dairy small ruminants, mainly because of its low incidence and because SCC is a relevant and simple indicator of intra-mammary infections. Work completed in France has developed two lines of ewes (experimental farm INRAE-La Fage) and goat (experimental farm INRAE-Bourges), a high line generated from sires with unfavourable EBVs for somatic cells and a low line generated from sires with favourable EBVs for somatic cells. For both sheep (Rupp et al., 2009) and goats (Rupp et al., 2019), the low line has the lowest SCC, the lowest incidence of clinical mastitis and the lowest incidence of chronic mastitis (abscesses or unbalanced udder) and subclinical mastitis (assessed by milk bacteriology).
Even though SCC is the established indicator for use in animal breeding, the use of the California Milk test (CMT) is a very good indicator of SCC for monitoring udder health in flock/herd management in dairy and meat-producing small ruminants.
Data recording
Somatic Cell Count (SCC)
Large scale somatic cell counting relies on the application of routine methods, such as fluoro- opto-electronic counting. The somatic cell counter must be properly calibrated against a reference and laboratories must frequently verify the calibration settings are still correct.
The design for recording SCC will depend upon the objective. For flock/herd management related to high bulk SCC, the whole flock/herd should be sampled and analysed to identify the animals with the highest SCC. For genetic purpose, simplified designs might be implemented.
In dairy species, somatic cell counting is achieved within the milk recording design and the sampling design, as for milk components such as fat and protein content. As in small ruminants, most of the designs are simplified ones compared to the A4 method (all daily milkings recorded, once a month) (see ICAR Guidelines Section 16: dairy sheep and goats), SCC are quite often available at one out of the two daily milkings. In this case, use of SCC must be handled accordingly.
As for milk composition, with the aim of simplifying and decreasing further the cost of recording, it is possible/recommended to measure SCC on only a part of the flock/herd (first parity or first two parities). It is also possible to go further in the simplification of the design, for example by sampling only a part of the lactation within a part-lactation sampling as proposed in the section 16 of the ICAR Guidelines. The genetic parameters of test-day and lactation mean for Somatic Cell Score (SCS - log-transformed SCC) show that the records of the middle of the lactation appear as the most representative of the whole lactation. Two to four individual samples per female and per lactation, collected monthly in the middle part of the lactation are highly correlated (around 0.98) with SCS determined from samples collected over the complete lactation (A4 method) but are hardly less heritable compared with the A4 homologous traits (negligible loss of precision for SCS) (Astruc and Barillet, 2004). The balance between cost and genetic efficiency, depending on the genetic correlations close to 1, is clearly in favour of the part-lactation sampling compared to A4 testing.
California Mastitis Test (CMT)
The California mastitis test is an animal-side diagnostic test that provides an estimate of the level of infection within a mammary gland. A sample of milk (~3ml) from each udder half is combined with an equal volume of reagent in a CMT paddle and mixed for 15 to 20 seconds. The reaction is scored based on the level of thickening of the mixture from zero (no thickening) consistent with no, or low, levels of infection, to four (gel formation with elevated surface) indicating high levels of infection.
A previous study (McLaren et al., 2018) has demonstrated the strong correlation between CMT score and SCC from samples collected from pedigree meat sheep in the UK.
Calculation of traits
Test-day SCC must be transformed to Somatic Cell Score (SCS) by the logarithmic transformation of Ali and Shook (1980) to achieve normality of distribution.
Example: SCS = log2+(SCC/100,000)+ 3
The table 4 gives correspondence between SCC and SCS
Table 4. Correspondence between somatic cell score and somatic cell count
| Somatic Cell Count (SCC) | Somatic Cell Score (SCS) |
| 12,500 | 0 |
| 25,000 | 1 |
| 50,000 | 2 |
| 100,000 | 3 |
| 200,000 | 4 |
| 400,000 | 5 |
| 800,000 | 6 |
| 1,600,000 | 7 |
SCS can be adjusted for days-in-milk (DIM). In this case, the adjustment procedure must be defined from a study based on healthy ewes/goats with enough number of test-days over the lactation. Then a lactation SCS (LSCS) may be calculated (case of lactation model in genetic evaluation).
LSCS can be computed as the weighted arithmetic mean of test-day SCS (adjusted or not for DIM). Weights are either 1 (equivalent to no weight) or r2, where r is the correlation between one measure and the mean of all other records.
Use for genetic analysis / genetic evaluation
Genetic model
The genetic model might include the following fixed effects:
- Flock x year (x parity)
- Month of lambing/kidding
- Age at lambing/kidding
- Number of lambs/kids born
Genetic parameters
Pooled estimates of heritability of somatic cell score gained by meta-analysis (Mucha et al., 2022) in dairy goats and sheep and meat sheep are shown in Table 5.
Table 5. Pooled estimates of heritability of somatic cell score from meta-analysis in dairy goats and sheep (Mucha et al., 2022)
| Trait1 | Species | Pooled h2 (± SE) | Min2 h2 | Max3 h2 | N obs | N studies |
| SCS | Goats
Sheep |
0.21±0.01
0.13±0.02 |
0.19
0.03 |
0.24
0.27 |
5
29 |
3
22 |
1Trait: SCS – somatic cell score
2Minimum h2 from individual studies included in meta-analysis
3Maximum h2 from individual studies included in meta-analysis
Table 6. Pooled estimates of genetic correlations (rg) between resilience (SCS, FEC) and efficiency (MY, FC, PC) traits from meta-analysis in dairy goats (Mucha et al., 2022)
| Traits1 | Pooled rg (± SE) | Min2 rg | Max3 rg | N obs | N studies |
| SCS & MY | 0.35±0.31ns | 0.00 | 0.59 | 3 | 3 |
| SCS & FC4 | -0.19±0.01 | -0.20 | -0.18 | 3 | 2 |
| SCS & PC | -0.06±0.05ns | -0.13 | 0.00 | 3 | 2 |
| FEC & MY | 0.17±0.35ns | -0.21 | 0.63 | 4 | 2 |
1Traits: SCS – somatic cell score, FEC – faecal egg count, MY – milk yield, FC – fat content, PC – protein content
2Minimum rg from individual studies included in meta-analysis.
3Mmaximum rg from individual studies included in meta-analysis.
nspooled correlations obtained from a simple random effects model as the three level meta-analysis model did not converge
Table 7. Pooled estimates of genetic correlations between resilience (SCS) and efficiency (MY, FY, PY, FC, PC) traits from meta-analysis in dairy sheep (Mucha et al., 2022)
| Traits1 | Pooled rg (± SE) | Min2 rg | Max3 rg | N obs | N studies |
| SCS & MY | -0.05±0.10ns | -0.30 | 0.23 | 16 | 11 |
| SCS & FC | 0.04±0.05ns | -0.16 | 0.16 | 8 | 8 |
| SCS & PC | 0.12±0.03 | 0.02 | 0.24 | 12 | 9 |
| SCS & FY | 0.11±0.15ns | -0.04 | 0.31 | 4 | 4 |
| SCS & PY | 0.17±0.10ns | 0.06 | 0.31 | 4 | 4 |
1Traits: SCS – somatic cell score, MY – milk yield, FY – fat yield, PY – protein yield, FC – fat content, PC – protein content
2Minimum rg from individual studies included in meta-analysis
3Maximum rg from individual studies included in meta-analysis
nsPooled correlations obtained from a simple random effects model as the three level meta-analysis model did not converge
ns – Pooled estimate did not differ significantly from zero
Table 8. Estimates of heritability of somatic cell score, clinical mastitis and CMT in meat and dairy and meat sheep (source Oget et al., 2019)
| Sheep | Breed | Trait1 | Heritability (±SE) | Reference |
| Dairy | Chios | CMT | 0.12±0.06 | Banos et al., 2017 |
| Meat | Belclare, Charollais, Suffolk, Texel,
Vendeen breeds |
CM | 0.04±0.03 | O’Brien et al., 2017 |
| Meat | Texel | SCS | 0.11±0.04 | McLaren et al., 2018 |
| Meat | Texel | CMT | 0.08-0.09±0.04 | McLaren et al., 2018 |
| Meat | Texel | CMT | 0.07 | Kaseja et al., 2023 submitted paper (SMARTER, D2.3) |
1CMT - California mastitis test, CM - Clinical mastitis (examination and palpation of the udder), SCS – Somatic Cell Score
Resistance to footrot
Definition, terminology, rationale
Footrot is caused by Dichelobacter nodosus and is a major cause of lameness in sheep. The disease is highly contagious and endemic in many countries that causes pain and welfare issues in affected animals. In addition to the direct impacts on time and veterinary / medicine costs, the disease has further, indirect, impacts through reducing fertility and milk supply.
The presence of footrot is assessed by inspection of the hooves of lame animals.
Data recording
Scoring methods
Each hoof is assessed individually and scored based on the five-point scale (used in UK): clean, unaffected hoof (score 0), mild inter-digital inflammation (score 1), inter-digital necrosis (score 2), under-running of the sole of the hoof (score 3) and fully under-run to the abaxial wall of the hoof (score 4) (Conington et al., 2008).
The sum of scores is calculated by adding all four scores (for each hoof), hence the animal can obtain the phenotype in a range from zero to 16.
In France, where footrot is usually not recorded, a simplified scoring system has been developed using a scale (0 normal and severity of lesions scored from 1 to 3) adapted from the Victorian Farmers Federation and Coopers Animal Health.
Additionally, the health of feet is assessed in France and the UK for other important hoof lesions including white line degeneration, contagious ovine digital dermatitis, horn growth, presence of abscess, granuloma, interdigital hyperplasia, and panaritium).
Calculation of traits
Sum of scores are log-transformed in order to normalise the data using the formula ln(Sum of scores + 1). The addition of one prevents to logarithm the value of sum of scores equal to zero. Each animal can obtain transformed score ranging between zero and 2.83.
Use for genetic analysis / genetic evaluation
Genetic model
The genetic model might include the following fixed effects:
- Age of the dam
- Scorer (if more than one)
- Vaccine status (if some animals treated with the vaccination against ovine foot-rot)
- Flock or Flock x Year interaction
Genetic parameters
The estimated heritability for UK meat sheep (Table 9) varies between 0.12 (Nieuwhof et al., 2008). to 0.23 (Kaseja et al., 2023, unpublished results) Table 9. Estimates of heritability of resistance to footrot in meat sheep breeds.
Table 9. Estimates of heritability of resistance to footrot in meat sheep breeds.
| Breed | Trait1 | Heritability (SE) | Reference |
| Texel | RF | 0.12(0.02) | Kaseja et al, 2023 in press |
| Scottish Blackface | CM | 0.19 to 0.23 | Kaseja et al., 2023 in press. |
| Scottish lambs | SCS | 0.12 | Nieuwhof et al., 2008 |
| Texel | CMT | 0.18 | Mucha et al., 2015 |
1RF - Resistance to footrot, CM - Clinical mastitis (examination and palpation of the udder), SCS – Somatic Cell Score, CMT - California mastitis test
Acknowledgements
We gratefully acknowledge the contributions to small ruminant health and disease guideline by the following people:
- Joanne Conington, SRUC, the UK
- Jean-Michel Astruc, IDELE, France
- Rachel Rupp, INRAE, France
- Beat Bapst, Qualitas AG, Switzerland
- Donagh Berry, TEAGASC, Ireland
- Beatriz Carracelas, INIA, Uruguay
- Antonello Carta, Agris Sardegna, Italy
- Gabriel Ciappesoni, INIA, Uruguay
- Arnaud Delpeuch, IDELE, France
- Frédéric Douhart, INRAE, France
- Karolina Kaseja, SRUC, the UK
- Ed Smith, The British Texel Sheep Society, the UK
- Flavie Tortereau, INRAE, France
- Stefen Werne, FiBL, Switzerland
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
This work also used deliverable from the Eurosheep project (Horizon 2020 under agreement N° 863056).
References
Aguerre, S., Jacquiet, P., Brodier, H., Bournazel, J.P., Grisez, C., Prévot, F., Michot, L., Fidelle, F., Astruc, J.M., Moreno, C.R. (2018). Resistance to gastrointestinal nematodes in dairy sheep: Genetic variability and relevance of artificial infection of nucleus rams to select for resistant ewes on farms. Vet. Parasitol. 256:16-23. https://doi.org/10.1016/j.vetpar.2018.04.004
Ali, A., Shook, G. (1980). An optimum transformation for somatic cell concentration in milk. J. Dairy Sci. 63:487-490.
Astruc J.M., Barillet F. (2004). Current challenge for milk recording in dairy sheep and goats: the simplification of milk sampling design for chemical composition and somatic cell counts of milk. Proceedings of the 34th ICAR session, Sousse, Tunisia, 31 May-3 June 2004.
Banos, G., Bramis, G., Bush, S.J., Clark, E.L., McCulloch, M.E.B., Smith, J., Schulze, G., Arsenos, G., Hume, D.A., Psifidi, A. (2017). The genomic architecture of mastitis resistance in dairy sheep. BMC Genom. 18 624.
Bell, A., McNally, J., Smith, D.V., Rahman, A., Hunt, P., Kotze, A.C., Dominik, S., Ingham, A. (2019). Quantification of Differences in Resistance to Gastrointestinal Nematode Infections in Sheep Using a Multivariate Blood Parameter. Vet. Parasitol. 270:31–39.
Bishop, S.C. (2012). Possibilities to Breed for Resistance to Nematode Parasite Infections in Small Ruminants in Tropical Production Systems. Animal., 6:741–747.
Bergonier, D., Crémoux, R. de, Rupp, R., Lagriffoul, G., Berthelot, X. (2003). Mastitis of dairy small ruminants. Vet. Res. 34:689–716.
Casu, S., Usai, M.G., Sechi, T. et al. Association analysis and functional annotation of imputed sequence data within genomic regions influencing resistance to gastro-intestinal parasites detected by an LDLA approach in a nucleus flock of Sarda dairy sheep. Genet Sel Evol 54, 2 (2022). https://doi.org/10.1186/s12711-021-00690-7
Conington, J., Hosie, B., Nieuwhof, G.J., Bishop, S.C., Bünger, L. (2008). Breeding for resistance to footrot- the use of hoof lesion scoring to quantify footrot in sheep. Vet. Res. Commun. 32(8):583-9. https://doi.org/10.1007/s11259-008-9062-x
Gruner, L., Bouix, J., Brunel, J.C. (2004). High genetic correlation between resistance to Haemonchus contortus and to Trichostrongylus colubriformis in INRA 401 sheep. Vet. Parasitol. 119:51–58.
Jacquiet, P., Salle, G., Grisez, C., Prevot, F., Lienard, E., Astruc, J.M, Francois, D., Moreno, C. (2015). Selection of sheep for resistance to gastro-intestinal nematodes in France: where are we and where are we going? 25th International Conference of the WAAVP, Liverpool, UK, 2015, 16-20 August
McLaren, A., Kaseja, K., Yates, J., Mucha, S., Lambe, N.R., Conington, J.(2018). New mastitis phenotypes suitable for genomic selection in meat sheep and their genetic relationships with udder conformation and lamb live weights. Animal. 12(12):2470-2479. doi: 10.1017/S1751731118000393.
Mucha, S., Bunger, L., Conington, J. (2015). Genome-wide association study of footrot in Texel sheep. Genetics Selection Evolution, 47 (1), pp.35. DOI 10.1186/s12711-015-0119-3
Mucha, S., Tortereau, F., Doeschl-Wilson, A., Rupp R., Conington, J. (2022). Animal Board Invited Review: Meta-analysis of genetic parameters for resilience and efficiency traits in goats and sheep. Animal. 16(3):100456. https://doi.org/10.1016/j.animal.2022.100456.
Nieuwhof, G.J., Conington, J., Bünger, L., Haresign, W., Bishop, S.C. (2008). Genetic and phenotypic aspects of resistance to footrot in sheep of different breeds and ages. Animal. 2(9):1289-1296. https://doi.org/10.1017/S1751731108002577
O’Brien, A.C., McHugh, N., Wall, E., Pabiou, T., McDermott, K., Randles, S., Fair, S., Berry, D.P. (2017). Genetic parameters for lameness, mastitis and dagginess in a multi-breed sheep population. Animal 11, 911–919. DOI: 10.1017/S1751731116002445
Oget, C., Tosser-Klopp, G., Rupp, R. (2019). Genetic and genomic studies in ovine mastitis. Small Ruminant Research 176, 55-64. https://doi.org/10.1016/j.smallrumres.2019.05.011.
Råberg, L, Sim, D., Read, A.F. (2007). ‘Disentangling genetic variation for resistance and tolerance to infectious diseases in animals’, Science. 318(5851), 812-814 DOI: 10.1126/science.1148526
Raynaud J.P. (1970). Etude de l’efficacité d’une technique de coproscopie quantitative pour le diagnostic de routine et le controle des infestations parasitaires des bovins, ovins, equines et porcins. Ann. Parasitol. 45: 321–342
Rupp, R., Bergonier, D., Dion, S., Hygonenq, M.C., Aurel, M.R., Robert-Granié, C., Foucras, G. (2009). Response to somatic cell count-based selection for mastitis resistance in a divergent selection experiment in sheep. J. Dairy Sci. 92, 1203–1219.
Rupp, R., Huau, C., Caillat, H., Fassier, T, Bouvier, F., Pampouille, E., Clément, V., Palhière, I., Larroque, H., Tosser-Klopp, G., Jacquiet, P., Rainard, P. (2019). Divergent selection on milk somatic cell count in goats improves udder health and milk quality with no effect on nematode resistance. J Dairy Sci. 102(6):5242-5253. doi: 10.3168/jds.2018-15664.
Sabatini, G.A., de Almeida Borges, F., Claerebout, E. et al. (2023). Practical guide to the diagnostics of ruminant gastrointestinal nematodes, liver fluke and lungworm infection: interpretation and usability of results. Parasit. Vectors. 16, 58. https://doi.org/10.1186/s13071-023-05680-w
Shaw, R.J., Morris, C.A., Wheeler, M., Tate, M., Sutherland, I.A. (2012). Salivary IgA: A Suitable Measure of Immunity to Gastrointestinal Nematodes in Sheep. Vet. Parasitol. 186, 109–117
Van Wyk, J.A., Bath, G.F. (2002). The FAMACHA© system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet. Res. 33:509–529.
Whitlock, H.V. (1948). Some modifications of the McMaster helminth egg counting technique and apparatus. J. Coun. Sci. Ind. Res. 21:177.
Annexes
Guidelines on survival recording of foetus and young in sheep and goats
Change Summary
| Date of change | Nature of Change |
| October 2024 | First version |
| December 2024 | Tracked change revisions by MS |
Introduction and scope
Introduction
Foetal and young survival are parameters linked to neonatal vigour scores, maternal and young behaviours, stress responses, immunity transfer and traits related to dam fertility and longevity. Minimising mortality, either in utero (e.g., embryo/foetus) or pre-weaning, are crucial to profitable small ruminant production systems. Survival depends on an interaction between the environment and behaviour of both, the ewe and the lamb. Ewes must give birth without complications and provide reliable source of colostrum along with mothering environment. Lamb must adapt to the extra-uterine environment, thermoregulate and be able to stand and suckle in a reasonably short period after birth (Brien et al., 2014; Plush et al., 2016). Despite this, pre- weaning survival in many species is far from ideal (Binns et al., 2002; Yapi et al., 1990, Chaarani et al., 1991, Green and Morgan, 1993, Nash et al., 1996). This can be particularly worse in small ruminant production systems which are typically more extensive and therefore prevailing weather conditions can be an additional stressor as well as predators. Moreover, the poly-ovulatory nature of species such as sheep and goats also predisposes such species to greater foetal and pre-weaned young losses (Scales et al., 1986).
Litter size can be determined using trans-abdominal ultrasonography of the uterine horns at ideally 40-70 days post-fertilisation. Good accuracy in determining foetal number has been reported from trans-abdominal ultrasonography (Taverne et al., 1985). The number of young eventually born can then be used to assess foetal loss since the time of scanning. At birth, young survival is usually based on dead or not in the first 24 h post-birth while stillborn individuals or those dead within 24 hours are usually defined as failed to survive. Young survival can also be considered as different age group categories until weaning – for example from 1 day to 7 days of age. Young animals (i.e., < 7 days) are greatest at risk of mortality (Binns et al., 2002) and tend to die of exposure to hypothermia, starvation, septicaemia, or repercussions from trauma suffered at birth.
Scope
The aim of the present section is to define approaches for the definition of foetal and lamb survival as well as the data editing and downstream analyses (including statistical models).
Definition, terminology, rationale
A plethora of different definitions exist depending on whether defined at the level of the individual (i.e., binary trait) or that of the litter. A non-exhaustive list is given below.
Foetal survival (at an individual level):
- Can be defined as a binary trait of 0 (died between scanning and birth) or 1 (survived between scanning and birth). A dummy ID for the dead foetus would need to be constructed but the parentage would still potentially be known (especially if generated from AI).
Foetal survival (at a litter level):
- Whether or not some foetal mortality has occurred defined as a binary trait (i.e., the number of individuals born is less than the number scanned in utero)
- Number of individual foetuses scanned alive (along with gestational age)
- Number of foetuses scanned minus the number that were born (dead or alive) – this is a measure of foetal mortality as opposed to survival and assumes stillborn young are considered in the definition of a young survival trait. It is a count trait
- The number of young born divided by the number of foetuses scanned (this is mortality rate figure but per little with a penalty on losses for smaller litter sizes).
Young survival (at an individual level):
- Can be defined as a binary trait of 0 (dead within 24 hours of birth) or 1 (alive after 24 hours of birth). The dead animal would need to receive an ID and can, of course, be genotyped to verify parentage (but also used for downstream genomic analyses discussed later).
Young survival (at a litter level):
- Number of lambs born alive (NLBA)
- Number of lambs dead within 24 hours of birth
- Number of lambs dead within 24 hours of birth divided by the total number of lambs born
Recording survival of foetuses and young in small ruminant
In all instances, accurate data is crucial. Data should be collected on the animal/dam itself (dead or alive) but also potential confounding effects that could be considered for inclusion in the statistical model as fixed effects. Examples include contemporary group (e.g., flock-date of scanning, flock-year-season of birth (for each NLB separately), ewe parity, litter size). Ideally also all individuals should be genotyped. Because the heritability of foetal or young animal mortality in small ruminants is relatively low (<0.1; Safari et al., 2005; Brien et al., 2014), a large number of records are required to achieve accurate genetic/genomic evaluations. Care should also be taken when interpreting the scoring (and the following genetic evaluations), some jurisdictions may record mortality rather than survival or may record mortality but propose genetic evaluations as survival (i.e., positive value is favourable).
Pregnancy scanning records
Ideally scanning should be undertaken 40 to 70 days post-fertilisation. This may be possible to (easily) achieve where extensive AI has been used but, otherwise, should ideally be 30 days after the last female has been marked as been served by natural mating. Skilled operators should be able to determine the number of foetuses from 30 to 100 days of gestation; usually only one operator will scan a flock on a given day so will be confounded with flock-date of scanning contemporary group. If AI is solely used or if single sire mated, then the parentage of the foetus should be known; if mob mated or single sire mated at AI, then superfecundation could cause a discrepancy in recorded sire.
Young survival
Young survival can be defined at birth, ideally as a binary trait as to whether the animal was born stillborn or died within 24 hours (survival = 0) or was still alive 24 hours after birth (survival = 1). If information is also available on the reason for death (i.e., autopsy results) then, where sufficient data exists for any one ailment, it could be analysed separately as separate traits. This could be particularly important for generating separate genetic evaluations for the main diseases thereby not only possibly increasing the heritability through more accurate data, but also provide genetic evaluations specific to individual ailments which could enable more selection pressure on these traits in situations where they are more impactful. Ideally a genotype of the dead animal should be generated. Any obvious external defects should be noted.
Ancillary information
Having ancillary information coinciding with an event is useful for several reasons:
- For helping data editing (e.g., comparing actual birth date to expected birth date based on recorded service information)
- For adjustment in the statistical model (e.g., dam parity)
- Understanding the risk factors associated with survival
- Enabling more precise estimates of correlations with other performance traits by having information on multiple features from the same animal
- Adjusting for possible selection in multi-trait genetic evaluation models
Possible ancillary information can be divided into those associated with 1) the past of prevailing environmental conditions, 2) the dam (or sire), or 3) the individual. Examples include:
1. Environment:
- Weather related factors (rainfall, temperature, wind including direction)
- Flock
- Date of scanning or date of birth
2. Dam
- Parity
- Age
- Breed
- Genotype
- Litter size
- Mating type (i.e., AI versus natural)
- Body condition score (change) and live-weight (change)
- Mothering ability
- Colostrum quality and yield
3. Individual
- Days since service (for foetal survival trait)
- Birthing difficulty
- Birth weight
- Gender
- Genotype
- Sire
- Autopsy results if possible
Use for genetic analysis / genetic evaluation
Data editing and statistical modelling
In order to estimate contemporary group effects well, the larger the contemporary group, the better the group estimates. Therefore, imposing a minimum contemporary group size prior to data analysis should be considered as should good genetic connectedness with other contemporary groups. Genetic connectedness can be an issue with small ruminant populations in particular, especially where natural mating prevails.
Data editing
Foetal survival - Each flock-scanning date can be firstly investigated at a macro level to measure ultrasound quality control. Simple cross-references between the number of females with scanning data versus those presented as well as the ID numbers of both is useful to ensure all data were properly recorded. High foetal mortality rates could simply be indicative of high foetal loss (e.g., abortions due to causes like chlamydial and toxoplasma) as well as poor operator competence – assessing the rate for individual operators across flocks (and time) could be useful to assess operator proficiency. A high proportion of litters where the number of young born (dead or alive) exceeds that recorded at scanning suggests a poor accuracy of recording. It should be considered to discard the data from that date but also to investigate the operator in more detail across other flocks, and irrespective, the scanning results from that litter at least should be discarded. The proportion of scanned litters with >3 detected foetuses should also be calculated; depending on the expected prolificacy of the animals (e.g., breed), then the appropriate editing of either the individual data points or the date in its entirety should be assessed.
Young mortality - A high incidence of young mortality per contemporary group could simply be a consequence of some underlying issue (e.g., predation, disease) or indeed a high fecundity rate; a low incidence of young could be indicative of a good stock person. Therefore, it can be difficult to distinguish between high and low quality data. Using guaranteed high quality and reliable data, it is possible to estimate the expected distribution of the incidence of young animal mortality for different population strata such as flock size, ewe age, breed, litter size. Using these distributions, the probability that the mean mortality for a contemporary group fits this distribution can be estimated and a decision made as to whether or not to include the data in the downstream analyses.
Statistical modelling
Lamb survival is a complex trait influenced by direct genetic, maternal genetic, and environmental effects. Due to discrete expression of phenotype (dead or alive: 0 or 1) it is described as a threshold trait (Falconer, 1989) that violates the assumption of normality, and therefore linear models are theoretically not appropriate for the analysis. However, examples from the literature analysed survival data and reported that linear models were marginally more accurate at predicting missing phenotypes than were logit-transformed alternatives and are convenient for interpretation on the observed scale (Matos et al., 2000; Everett-Hincks et al., 2014; Cloete et al. 2009; Vanderick et al., 2015;).
Random effects considered in the statistical model are direct and maternal genetic effects and maternal permanent environment across parities. A litter permanent environmental effect should also be considered as a random effect where the trait is that of the individual (and not the ewe). Traditionally, relationships were accounted for though the pedigree data, however this can often now be supplemented with genome-wide genotype information to generate a H matrix (i.e., combines genomic and ancestry information). Whether the estimation of these additional covariance components improve the fit to the data can be deduced by a likelihood ratio test but ideally a metric such as the AIC or BIC to account for the increased complexity of the model.
The choice of environmental factors included in the model will depend on the population being studied and considers the following fixed effects:
- Contemporary group (e.g., flock-date of scanning for foetal survival and flock-year-season of birth or flock-year-season-birth rank of birth)
- Lamb gender (may not be possible for foetal survival trait)
- Dam parity
- Mating type (i.e., AI versus natural)
- Dam age nested within parity
- Day of gestation (for foetal survival) if available or defined as a categorical variable
- Litter size (at scanning or birth) or birth type (single and multiple)
- Heterosis and recombination loss of the dam and foetus/young
- Inbreeding coefficient of the dam and foetus/young
- Age of the sire
- Breed composition of the dam and foetus/young
Adjusting for the effects such as dystocia or birth weight, may not be appropriate in the statistical model for young survival as they are likely to be genetically correlated with survival and thus may remove some of the true genetic variance – nonetheless, the eventual decision will be based on the genetic evaluation system employed and how the economic value on the traits within the overall breeding objectives are constructed.
Genomic association analyses
Where genotypes are available, then a genome-wide association study (or candidate gene study) can be undertaken (Esmaeili-Fard et al., 2021). Although it is not possible to have the genotype of the aborted foetus, it could still be possible to undertake a genomic analysis especially by focusing on the genotype/haplotype of the living animals versus the expectation based on the genotype/haplotype of the parents (Ben Braiek et al., 2021).
Acknowledgements
We gratefully acknowledge the contributions to these recording of survival of foetus and young guidelines by the following people:
- Donagh Berry, TEAGASC, Ireland
- Joanne Conington, SRUC, the UK
- Maxime Ben Braiek, INRAE, France
- Arnaud Delpeuch, IDELE, France
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Ben Braiek, M., Fabre, S., Hozé, C., et al. (2021). Identification of homozygous haplotypes carrying putative recessive lethal mutations that compromise fertility traits in French Lacaune dairy sheep. Genet. Sel. Evol. 53:41. https://doi.org/10.1186/s12711-021-00634-1
Binns, S.H., I.J.Cox, S. Rizvi, L.E.Green. (2002). Risk factors for lamb mortality on UK sheep farms. Prev.Vet. Med.. 52:287-303.
Brien, F.D., Cloete, S.W.P., Fogarty, N.M., Greeff, J.C., Hebart, M.L., Hiendleder, S., Hocking Edwards, J.E., Kelly, J.M., Kind, K.L., Kleeman, D.O., Plush, K.L., Miller, D.R (2014). A review of genetic and epigenetic factors affecting lamb survival. Anim. Prod. Sci. 54:667–693.
Chaarani, B., Robinson, R.A., Johnson, D.W. (1991). Lamb mortality in Meknes Province (Morocco). Prev. Vet. Med. 10:283-298.
Cloete, S.W.P., Misztal, I., Olivier, J.J. (2009). Genetic parameters and trends for lamb survival and birth weight in a Merino flock divergently selected for multiple rearing ability. J. Anim. Sci. 87:2196–2208. doi:10.2527/jas.2008-1065.
Esmaeili-Fard, S.M., Gholizadeh, M., Hafezian, S.H., Abdollahi-Arpanahi, R. (2021) Genes and Pathways Affecting Sheep Productivity Traits: Genetic Parameters, Genome-Wide Association Mapping, and Pathway Enrichment Analysis. Front. Genet. 12:710613. doi:10.3389/fgene.2021.710613.
Everett-Hincks, J.M., Mathias-Davis, H.C,, Greer, G.J., Auvray, B.A., Dodds, K.G. (2014). Genetic parameters for lamb birth weight, survival and deathrisk traits. J. Anim. Sci. 92:2885–2895. doi:10.2527/jas.2013-7176.
Falconer, D.S. (1989). Introduction to Quantitative Genetics.’ (Longmans Green/John Wiley & Sons: Harlow, Essex, UK).
Green, L.E., Morgan, K.L. (1993). Mortality in early born, housed lambs in south-west England. Prev. Vet. Med. 17:251-261.
Matos, C.A.P., Thomas, D.L., Young, L.D., Gianola, D. (2000). Genetic analyses of lamb survival in Rambouillet and Finnsheep flocks by linear and threshold models. Anim. Sci. 71:227–234. doi:10.1017/S1357729800055053.
Nash, M.L., Hungerford, L.L., Nash, T.G., Zinn, G.M. (1996). Risk factors for perinatal and postnatal mortality in lambs. Vet. Rec. 139:64-67.
Plush, K.J., Brien, F.D., Hebart, M.L., Hynd, P.I. (2016). Thermogenesis and physiological maturity in neonatal lambs: a unifying concept in lamb survival. Anim. Prod. Sci. 56:736–745. https://doi.org/10.1071/AN15099.
Safari, E, Atkins, K.D., Fogarty, N.M., Gilmour, A.R (2005). Analysis of lamb survival in Australian Merino. Proceedings of the Association for the Advancement of Animal Breeding and Genetics. 16:28–31.
Scales, G. H., Burton R. N., Moss, R. A. (1986). Lamb mortality, birthweight, and nutrition in late pregnancy. N. Z. J. Agric. Res. 29:1.
Taverne, M.A.M. Lavoir, M.C., van Oord R., van der Weyden, G.C. (1985) Accuracy of pregnancy diagnosis and prediction of foetal numbers in sheep with linear‐array real‐time ultrasound scanning. Vet. Q. 7:(4)256-263, DOI: 10.1080/01652176.1985.9693997.
Vanderick, S., Auvray, B., Newman, S.A., Dodds, K.G., Gengler, N., EverettHincks, J.M. (2015). Derivation of a new lamb survival trait for the New Zealand sheep industry. J. Anim. Sci. 93:3765–3772. doi:10.2527/jas.2015-9058.
Yapi, C.V., Boylan, W.J., Robinson, R.A. (1990). Factors associated with causes of preweaning lamb mortality. Prev. Vet. Med., 10:145-152.
The technical references (papers cited or used) are documented in each piece of recommendations.
Recording behavioural traits in sheep and goat
Change summary
| Date of change | Nature of Change |
| October 2024 | First version |
| November 2024 | Tracked change revisions by JC |
| December 2024 | Tracked change revisions by MS |
Introduction and scope
Introduction
Genetic selection including behavioural traits could be an advantageous strategy for improving robustness and welfare of farm animals in various farming conditions by minimizing unsuitable responses to changes in their social and physical environment, limiting an excessive fear of humans and improving sociability (Mignon-Grasteau et al., 2005). Farm animals are social and gregarious, and relational behaviours are essential for ensuring social cohesion, social facilitation, offspring survival and docility toward humans. Breed differences and genetic variation within breed have been reported in lambs for early social behaviours and found to be heritable, and associated with some QTL, suggesting such behaviours could be selected early (Boissy et al., 2005; Beausoleil et al., 2012; Hazard et al., 2014; Cloete et al., 2020). In addition, such early social reactivity of lambs towards conspecifics or humans was identified as a robust trait and that selection for early social reactivity of lambs towards conspecifics or humans is feasible (Hazard et al., 2016; 2022).
The behaviour of both ewes and lambs, and their interaction at lambing, have been widely described. Such behaviour is important for the survival of the offspring, especially in extensive farming conditions as reviewed by Dwyer et al. (2014). Moreover, it has been shown that primiparous ewes are more prone to abandon their lambs due to their lack of maternal experience (Dwyer, 2008) and that lamb survival at birth is lowly heritable (Brien et al., 2014). Taken together these factors could hinder the development of extensive farming systems. Genetic selection on maternal attachment traits could therefore be advantageous to improve offspring survival and growth, and reduce labour, as suggested by Mignon-Grasteau et al. (2005). Genetic variations in maternal behaviour between breeds of sheep have been well documented (for review see: Dwyer, 2008; von Borstel et al., 2011) while little was known about within-breed genetic variability and even less about maternal reactivity traits. We hypothesized that maternal attachment to the litter has a genetic component in sheep, and we recently reported that as expected the maternal reactivity at lambing is a heritable trait (Hazard et al., 2020;2021).
Grazing behaviour is also important for animals raised in extensive production systems because it can support adaptability to changing environments. In particular, small ruminants reared in semi-extensive systems face many environmental and welfare challenges that are difficult to quantify. The evidence in the literature suggests that there are differences in grazing behaviour between and within breeds of sheep (Simm et al., 1996; Brand, 2000). The notion is that natural selection combined with subjective artificial selection have led to some animals being more adaptive to extensive conditions. In this regard, genetic variation may exist for key grazing behaviour traits (Simm et al., 1996; Dwyer et al., 2005), but relevant literature is scarce. During the SMARTER H2020 project, a study was performed on grazing behaviour of the indigenous Boutsko Greek mountainous sheep breed, which is reared semi-extensively. The results showed that duration of grazing and speed are heritable traits (Vouraki et al., 2025).
Acronyms used in these guidelines
- AT Arena Test
- CT Corridor Test
- GPS Global Positioning System
- LS Lambing Site
- PCA Principal Component Analysis
Scope
The aim of the present report is i) to define the behavioural traits of interest, ii) to describe approaches for behavioural measurements, iii) to describe their use for genetic analysis and evaluation.
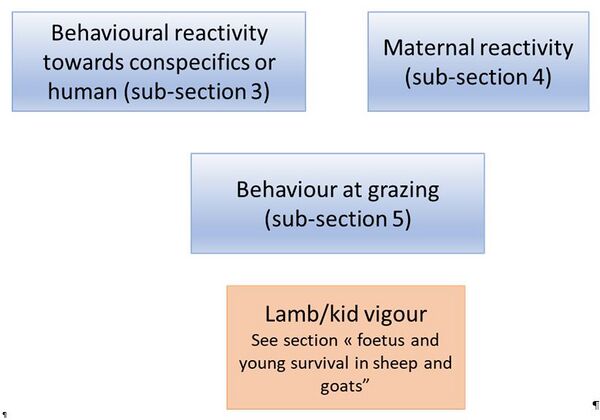
To-date, the present guidelines describe 3 groups of traits related to behaviour:
- Behavioural reactivity towards conspecifics or humans
- Maternal reactivity
- Behaviour at grazing
Kid/lamb vigour is a relevant behavioural trait, but this trait is tackled within the section “foetus and young survival in sheep and goats” of the guidelines.
Most of the work undertaken on behaviour concerned sheep. This has been particularly the case in SMARTER. Most of the recommendations might be applied to goats as well. Nevertheless, we will use the ovine terms in the guidelines below.
Behavioural reactivity towards conspecifics or humans
Definition, terminology, rationale
Behavioural reactivity towards conspecifics (i.e. sociability):
It is the social motivation of the lambs to join their conspecifics in response to social isolation with or without presence of a motionless human. Expression of higher levels of a panel of behaviours, including vocalisations and locomotion, is hypothesised as an active way to maintain social link with conspecifics.
Behavioural reactivity towards humans (i.e. docility):
It is the reactivity of isolated lambs to a walking human. Higher flight distance between the lamb and a human indicates a lower docility toward a human.
Behavioural reactivity towards conspecifics and humans are measured in standardised behavioural tests (arena and corridor tests, described below).
Higher sociability and/or docility towards humans may improve adaptation of sheep to harsh environments through social facilitation (i.e. transmission of feeding preferences…), social cohesion (i.e. transhumance…) and reactivity to handling. Consequently, improving such behavioural traits may improve welfare, production, and labour of shepherd.
Data recording
Behavioural tests
The test described below have been implemented in France. It must be considered as a possible test, as others can be described later and enrich these guidelines.
Lambs must be individually exposed just after weaning (i.e. approximately 10 days after weaning) to two behavioural tests. The delay between weaning and behavioural tests must be sufficient for the change of social preferences of lambs for their dam to conspecifics.
The arena test (AT) consists of two successive phases evaluating 1) reactivity to social isolation (AT1), 2) the motivation of the lamb towards conspecifics in presence of a motionless human (AT2). The arena test is performed indoors. The arena test pen consists in an unfamiliar enclosure virtually divided into 7 zones as described in detail by Ligout et al. (2011) (Figure 1). On one side of the enclosure (i.e. at the opposite of the entrance), a grid separates the tested lamb from another smaller pen containing 3 or 4 conspecifics. The first phase of the test (arena test phase 1, AT1) starts once the tested animal joins its flock-mates located behind a grid at the opposite side of the arena (time duration for joining: lower than 15 sec). No behavioural recording is performed during the joining. At this time, an opaque panel is pulled down (from the outside of the pen) between the flock-mates and the tested lamb to prevent visual contact. After one minute the phase 1 stops and the panel is pulled up so the lamb can see its flock-mates again. Once the lamb has returned near to its flock-mates, or after 1 minute if the lamb did not do so, a non-familiar human slowly enters the arena through a door located near the pen of the flock-mates and stood 20 cm in front of the grid separating the arena from the lamb’s flock-mates. The second phase (arena test phase 2, AT2) starts once the human is in place and lasts for a further 1 minute.

The corridor test (CT) consists of two successive phases evaluating 1) reactivity to social isolation (CT1) and 2) reactivity to an approaching human (CT2). The test pen consists in a closed, wide rectangular circuit and has been described in detail by Boissy et al. (Boissy et al., 2005) (Figure 2). The first phase (corridor test phase 1, CT1) starts when the lamb enters the testing pen and lasts for 30 seconds. After that time a non-familiar human enters the testing pen and the second phase (corridor test phase 2, CT2) starts and lasts 1 minute. During this phase, the human walks at a regular speed through the corridor (the corridor is divided into 6 virtual zones and one zone is crossed every 5 seconds) until two complete tours has been achieved.
Behavioural traits
Several behaviours are measured during behavioural tests: vocalisations (i.e. frequency of high- pitched bleats), locomotion (i.e. number of virtual zones crossed), the proximity score (i.e. weighting of time spent in virtual zones, a high score indicated a high duration spent close to conspecifics and a human).
An investigator counts the lamb’s vocalisations directly during the tests, from outside the pen using a laptop: number of times the animal bleats with an open mouth (high bleats, AT1/2- HBLEAT, CT1-HBLEAT). Locomotor activity is assessed by measuring the number of virtual zones crossed during arena test phases 1 and 2 (AT1/2-LOCOM) and corridor test phase 1 (CT1- LOCOM). This behaviour can be assessed using video recording or using infrared cells regularly positioned along the AT to detect displacement. The proximity to flock-mates and the human during AT2 is calculated by weighting of time spent in virtual zones (i.e. a high score indicated a high duration spent close to conspecifics and a human).
During CT2, every five seconds throughout this phase, an investigator records with a laptop the zones in which the human and the animal are located. In addition, the walking human records with a stopwatch the total duration during which the head of the lamb is visible. The mean flight distance (DIST) separating the human and the lamb (i.e. knowing the length of each virtual zone) and the time during which the human sees the lamb (SEEN) is measured in CT2.
Calculation of traits
Deviations from normality of row data must be tested using relevant statistical tests (e.g. the Kolmogorov–Smirnov test). Several raw measures must be transformed in order to minimise major deviations from the normal distribution. Square root transformation is applied to AT1/2- HBLEAT, CT1-HBLEAT. A multivariate analysis may be performed to take into account the multidimensional aspect of behavioural responses. Results of principal component analysis (PCA) indicate that the main principal components is structured mainly with similar behaviour (i.e. higher weight of similar behaviours for the different tests on the same component). Consequently, three synthetic variables may be constructed using PCA. Each PCA is performed for a set of similar behavioural variables across the behavioural tests. The first component of each PCA, explaining the largest part of total variance, is defined as a synthetic variable. Two synthetic variables are specific to the reactivity to social isolation: high bleats (HBLEAT, using AT1/2-HBLEAT and CT1- HBLEAT), locomotion (LOCOM, using AT1/2-LOCOM and CT1-LOCOM). One synthetic variable is specific to the reactivity to an approaching human: the tolerance to being approached when the lamb is free to flee (HUMAPPRO, using CT2-DIST and CT2-SEEN).
Use for genetic analysis and genetic evaluation
Genetic analyses and genetic evaluation can be performed on single traits and synthetic variables. Genetic analyses (estimation of (co)variance components and prediction of breeding values) for quantitative behavioural traits may be implemented with a mixed model methodology in animal model. Random effects should include:
- a direct additive genetic effect of the animal (i.e. lamb),
- a maternal permanent environment effect (i.e dam), that describes lamb phenotypic variation caused by the environment of the ewe
- a litter permanent environment effect, that accounts for phenotypic variation caused by the environment of the litter of the lamb being tested.
All relevant fixed effects and interactions should be included in the model. Factors that could be considered include:
- a combination of the litter size at lambing and the number of lambs suckled with their dam
- sex, age, live weight of the lamb,
- dam parity and/or age of dam nested withing parity if needed contemporary group (e.g., depending on the data collection: flock-year-season, grazing location…)
Maternal reactivity
Definition, terminology, rationale
- Behavioural reactivity at lambing (i.e. maternal reactivity). It is the social motivation or attachment of the ewe for the litter expressed in response to an approaching human, or the withdrawal of the litter with or without presence of a human. Expression of higher levels of a panel of behaviours, including maternal behaviour scores, vocalisations and locomotion, is hypothesised as an active way to maintain social link with lambs.
Maternal reactivity is measured in standardised behavioural tests (a scoring test outdoors, an arena test indoors, described in the controlled test below) or a maternal behaviour score (MBS) designed for use in extensive sheep systems as described by O’Connor et al (1985), the genetic basis of which was reported by Lambe et al., 2001 for Scottish Blackface sheep.
Higher maternal reactivity may improve adaptation of sheep to harsh environments through a higher behavioural autonomy at lambing and a reducing dependency to the support provided by shepherds. Consequently, improving such behavioural traits may improve welfare, production, and labour of the shepherd.
Data recording
Behavioural tests
The controlled test described below have been implemented in France. It must be considered as a possible test, as others can be described later and enrich these guidelines. Ewes are individually exposed to two behavioural tests: a scoring test performed just after lambing, outside at the lambing site, and then an arena test performed indoor, one day after lambing. The second test is performed after the bonding period needed to establish the social link between ewes and lambs and which occurs generally within the first twelve hours after lambing (Keller et al, 2003).
Scoring test at lambing site: Maternal reactivity is assessed outside at the lambing site approximately 2 hours after lambing, only on ewes that lambed during daylight when the shepherd approaches the lambing ewes to catch lambs for weighing and identification. Scoring at lambing is not performed in the following situations: if the location of the lambing site does not readily facilitate the testing procedure, if there are perturbations of scoring due to interference by other ewes, for sanitary reasons that could affect behaviours (including difficult lambing, death of all lambs of a litter). Measurement of maternal reactivity at the lambing site (LS) consists of two successive phases: (1) when the shepherd approaches the lambs; and (2) the capture and displacement of the lambs by the shepherd. In the first phase (LS1), the shepherd stands approximately 15 meters away from the lambing spot and approaches the ewes and the lambs at a regular speed (1 m/s). In the second phase (LS2), the shepherd catches all the lambs at the same time and moves away from the lambing spot in the same direction as that of the approach, stopping at the starting point where he places the lambs back on the ground and then moves 15 meters away to allow the ewe to restore contact with her lambs. This second phase of the test is not applied to ewes that flee at the approach of the shepherd and do not return within 60 seconds after the end of LS1.
Arena test: After lambing, all the ewes and lambs (both day and night births) are transferred to a shelter close to the place of lambing and penned individually for few hours. They are then moved to a collective pen until the next day when they are tested in the arena test (24h ± 6h after lambing). The arena test (AT) is performed indoors and adapted from the original test developed by Boissy and colleagues (2005) to investigate social attachment in sheep (Ligout et al., 2011). In the present study, the test consists of three successive phases evaluating the ewe’s 1) attraction to her litter, 2) reactivity to social separation from her litter, and 3) reactivity to a conflict between social attraction to her litter and avoidance of a motionless human. The test pen consists of an unfamiliar enclosure virtually divided into 7 zones (zone 7 being the zone nearest to the litter). On one side of the enclosure, a grid separates the tested ewe from another smaller pen containing her lamb(s). The first phase of the test (AT1) starts when the tested ewe enters the arena and lasts for 30 s. Then, a remotely controlled opaque panel is pulled down in front of the grid to prevent visual contact between the tested ewe and her lambs. The second phase (AT2), during which the tested ewe is separated from her lambs, lasts 1 min. Finally, the panel is raised so the tested ewe can see her lamb(s) again. Once the ewe has returned near to her lamb(s), a non-familiar shepherd slowly enters the arena through a door located near the grid separating the arena from the litter and stands 20 cm in front of the grid. The third phase of the test (AT3) starts once the shepherd is in place and lasts for 1 min.
Behavioural traits
Scoring test at lambing site: A scoring system, close to those defined by O’Connor et al. (1985), and further validated for hill sheep by Lambe et al. (2001) for use in animal breeding programmes to enable many animals to be scored relatively quickly and easily in extensive sheep systems. The simple scoring system measures maternal reactivity described for each of the two phases described above. In LS1, a maternal behaviour score (LS1-MBS) is recorded on a 5-point scale as follows: 1 - ewe flees and does not return to the lambs within 60 s; 2 - ewe retreats (i.e., at least 2-3 m) but comes back to her lambs within 60 s; 3 - ewe retreats with at least one lamb and comes back; 4 - ewe retreats and returns repeatedly; 5 - ewe stays close to the lambing spot. In LS2, a second maternal behaviour score (LS2-MBS) is recorded on a 4-point scale as follows: 1 - ewe flees; 2 - ewe stays close to the lambing spot, 3 - ewe follows but from a distance (i.e., 1 to 2 m), 4- ewe follows, staying close to the shepherd (i.e., less than 1 m).
Arena test: Locomotor activity and localisation are analysed from the video footage or infrared cells (as described above). Locomotor activity is assessed by measuring the number of zones crossed during the 3 phases (AT1/2/3-LOCOM). The time spent in each zone is recorded. The ewe’s proximity to the litter and/or the human during phases 1 and 3 (AT1/3-PROX) is calculated using the following formula:
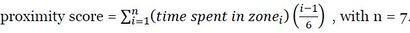
Two types of vocalisations are recorded manually during the test with an electronic device: number of high-pitched bleats are recorded when the animal bleats with an open mouth (AT1/2/3-HBLEAT) and number of low-pitched bleats are recorded when the animal bleats with a closed mouth (AT1/2/3-LBLEAT).
Calculation of traits
Logarithmic transformation is applied to AT1/2/3-LBLEAT to minimise major deviations from the normal distribution. All other elementary variables described above are directly used for genetic analyses.
Use for genetic analysis / genetic evaluation
The (co)variance components for quantitative behavioural traits can be estimated by restricted maximum likelihood (REML) methodology applied in an animal model. The (co)variance components for categorical behaviours can be estimated by MCMC and Gibbs sampling methods using a threshold model (Gilmour et al., 2009).
Assuming that all ewes are measured every year, the analyses assume a repeatability model with behaviour measured across productive cycles considered to be the same trait with a constant variance. Random effects typically include a direct additive and permanent environmental genetic effects of the animal (i.e., ewe).
All relevant fixed effects and interactions should be included in the model. Factors that could be considered can include:
- The litter size at lambing.
- Dam parity or age or age of the dam nested within parity (if significant).
- Contemporary group (e.g., depending on the data collection: flock-year-season effect…).
Behaviour at grazing
Definition, terminology, rationale
Grazing behaviour is a complex combination of various movements and activities of animals in different spatial-temporal scales (Andriamandroso et al, 2016). Indicative traits related to grazing behaviour include:
- Duration of grazing
- Distance walked
- Speed
- Altitude difference
- Elevation gain/loss
- Energy expenditure at grazing
A better understanding of the phenotypic and genetic background of grazing behaviour traits could help towards the development of appropriate breeding programmes to increase adaptation to extensive rearing conditions. However, recording of such traits is challenging. The use of new technologies such as global positioning systems (GPS) could help towards efficiently monitoring grazing behaviour (Homburger et al., 2014; Feldt and Schlecht, 2016).
Data recording
The following guidelines for recording grazing behaviour traits of sheep are based on a study implemented in Greece (Vouraki et al., 2025). Specifically, in the latter study, grazing behaviour of Boutsko sheep reared semi-extensively in mountainous regions was monitored using GPS technology. Moreover, phenotypic and genetic parameters for key grazing behaviour traits were estimated. These guidelines could be enriched in the future based on other relevant studies.
Monitoring of sheep grazing behaviour is performed using appropriate GPS devices attached on designated collars (Figure 3). Rotational monitoring of animals can be applied to reduce the number of devices needed. Selected GPS devices should be of low weight in order to be accepted by the animals without any obvious irritation. Batteries with extended life should be used to provide sufficient energy for GPS tracking for as many as possible consecutive days. In the aforementioned study, “Tractive GPS” devices (Tractive, Pasching, Austria) were used that weighed 28 grams. GPS tracking of each animal was performed for 4-10 days at 2-60 minutes intervals; number of tracking days and intervals were based on available signal and animal movement.
GPS generated data of each animal for the total tracking period are exported in .gpx format. In the case of “Tractive GPS”, the location history function of MyTractive web app (https://my.tractive.com/#/) is used to export recorded data. Then, the exported files are split by date using a designated software such as GPSBabel (version 1.8.0). For each animal, daily routes and corresponding GPS data can be visualized and extracted using appropriate software such as Viking GPS data editor and analyser (version 2.0).
Recorded grazing behaviour traits via these devices include duration of daily grazing (min), distance (km), speed (km/hour), minimum and maximum altitude, and total elevation gain. Other useful metrics including number and average distance between tracking points, tracking duration and route followed by the animals should also be extracted to be used in ensuing analyses.

Calculation of traits
Based on minimum and maximum altitude, altitude difference is calculated. Moreover, energy expenditure for walking can be estimated using the following formula of AFRC (Alderman and Cottrill, 1993):
EE= (0.0026×HD+0.028×VD)×BW
where:
EE = energy expenditure for walking (MJ);
HD = horizontal distance (km, calculated as the difference between distance and elevation gain); VD = vertical distance (km, corresponding to elevation gain);
BW = body weight (kg).
Quality control of GPS generated phenotypes is necessary to sense-check the data for extreme values and errors. Specifically, limits are set for minimum and maximum altitudes to reflect the real altitude of the region being studied. Tracking points beyond these limits are then removed from the corresponding .gpx files and data are re-calculated. Moreover, daily records for which GPS tracking of animals had stopped before returning to their shed, must be excluded. Finally, if needed, grazing behaviour traits should be logarithmically transformed to ensure normality of distribution prior to analyses.
Use for genetic analysis / genetic evaluation
(Co)variance components of grazing behaviour phenotypes and relevant breeding values (EBVs) can be estimated by restricted maximum likelihood methodology applied to an animal mixed model that can include the following random and fixed effects:
Random effects: additive genetic effect and permanent environmental effect of the animal
The relevant fixed effects may include:
- Farm
- Number of GPS tracking points
- Tracking duration
- Distance between tracking points
- Climatic parameters (e.g. temperature-humidity index)
- Sampling time
It may also be desirable to include social grouping (if known), as this can also affect individual animal behaviours.
Acknowledgements
We gratefully acknowledge the contributions to these recording of behaviour guidelines by the following people:
- Dominique Hazard, INRAE, France
- Angeliki Argyriadou, University of Thessaloniki, Greece
- Georgios Arsenos, University of Thessaloniki, Greece
- Alain Boissy, INRAE, France
- Vasileia Fotiadou, University of Thessaloniki, Greece
- Sotiria Vouraki, University of Thessaloniki, Greece
- Joanne Conington, SRUC, the UK
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Alderman, G., Cottrill, B. Energy and Protein Requirements of Ruminants. In An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients; CAB International: Wallingford, UK, 1993.
Andriamandroso, A., J. Bindelle, B. Mercatoris, F. Lebeau (2016). A review on the use of sensors to monitor cattle jaw movements and behavior when grazing. Biotechnologie, Agronomie, Société et Environnement, 20.
Beausoleil, N. J., D. Blache, K. J. Stafford, D. J. Mellor, and A. D. L. Noble. (2012). Selection for temperament in sheep: Domain-general and context-specific traits. Appl. Anim. Behav. Sci. 139:74–85.
Boissy, A., Bouix, J., Orgeur, P., Poindron, P., Bibe, B., & Le Neindre, P. (2005). Genetic analysis of emotional reactivity in sheep: effects of the genotypes of the lambs and of their dams. Genetics Selection Evolution, 37, 381-401. doi:10.1051/gse:2005007
Brand, T. S. (2000). Grazing behaviour and diet selection by Dorper sheep. Small Ruminant Research, 36(2), 147-158.
Brien, F. D., Cloete, S. W. P., Fogarty, N. M., Greeff, J. C., Hebart, M. L., Hiendleder, S., . . . Miller, D. R. (2014). A review of the genetic and epigenetic factors affecting lamb survival. Animal Production Science, 54, 667-693. doi:10.1071/an13140
Cloete, S. W. P., Burger, M., Scholtz, A. J., Cloete, J. J. E., Kruger, A. C. M., & Dzama, K. (2020). Arena behaviour of Merino weaners is heritable and affected by divergent selection for number of lambs weaned per ewe mated. Applied Animal Behaviour Science, 233. doi:10.1016/j.applanim.2020.105152
Dwyer, C. M., Lawrence, A. B. (2005). A review of the behavioural and physiological adaptations of hill and lowland breeds of sheep that favour lamb survival. Applied animal behaviour science, 92(3), 235-260.
Dwyer, C. M. (2008). Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management. Journal of Animal Science, 86, E246-E258. doi:10.2527/jas.2007-0404
Dwyer, C. M. (2014). Maternal behaviour and lamb survival: from neuroendocrinology to practical application. animal, 8, 102-112. doi:doi:10.1017/S1751731113001614
Feldt, T., Schlecht, E. (2016). Analysis of GPS trajectories to assess spatio-temporal differences in grazing patterns and land use preferences of domestic livestock in southwestern Madagascar. Pastoralism, 6(1), 1-17.
Gilmour, A. R., Gogel, B. J., Cullis, B. R., & Thompson, R. (2009). ASReml User Guide Release 3.0 VSN International Ltd, Hemel Hempstead, HP1 1ES, UK. www.vsni.co.uk.
Hazard, D., Moreno, C., Foulquié, D., Delval, E., François, D., Bouix, J., Boissy, A. (2014). Identification of QTLs for behavioral reactivity to social separation and humans in sheep using the OvineSNP50 BeadChip. BMC Genomics, 15, 778. doi:10.1186/1471-2164-15-778
Hazard, D., Bouix, J., Chassier, M., Delval, E., Foulquie, D., Fassier, T., Boissy, A. (2016). Genotype by environment interactions for behavioral reactivity in sheep. Journal of Animal Science, 94, 1459-1471. doi:10.2527/jas2015-0277
Hazard, D., Macé, T., Kempeneers, A., Delval, E., Foulquié, D., Bouix, J., & Boissy, A. (2020). Genetic parameters estimates for ewes’ behavioural reactivity towards their litter after lambing. Journal of Animal Breeding and Genetics, n/a. doi:10.1111/jbg.12474
Hazard, D., Kempeneers, A., Delval, E., Bouix, J., Foulquie, D., & Boissy, A. (2021). Maternal reactivity of ewes at lambing is genetically linked to their behavioural reactivity in an arena test. Journal of Animal Breeding and Genetics, 139, 193-203. doi:10.1111/jbg.12656
Hazard, D., E. Delval, S. Douls, C. Durand, G. Bonnafe, D. Foulquié, D. Marcon, C. Allain, S. Parisot, A. Boissy (2022). Divergent genetic selections for social attractiveness or tolerance toward humans in sheep. WCGALP 2022
Homburger, H., Schneider, M. K., Hilfiker, S., Lüscher, A. (2014). Inferring behavioral states of grazing livestock from high-frequency position data alone. PLoS One, 9(12), e114522.
Keller, M., Meurisse, M., Poindron, P., Nowak, R., Ferreira, G., Shayit, M., & Levy, F. (2003). Maternal experience influences the establishment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Developmental Psychobiology, 43, 167-176. doi:10.1002/dev.10130
Lambe, N R; Conington, J; Bishop, S C; Waterhouse, A; Simm, G (2001). A Genetic Analysis of maternal behaviour score in Scottish Blackface sheep. Animal Science 72: p415-425. Doi:10.1017/s1357729800055922.
Ligout, S., Foulquie, D., Sebe, F., Bouix, J., & Boissy, A. (2011). Assessment of sociability in farm animals: the use of arena test in lambs. Applied Animal Behaviour Science, 135, 57-62. doi:10.1016/j.applanim.2011.09.004
Mignon-Grasteau, S., Boissy, A., Bouix, J., Faure, J.-M., Fisher, A. D., Hinch, G. N., . . . Beaumont, C. (2005). Genetics of adaptation and domestication in livestock. Livestock Production Science, 93, 3-14. doi:10.1016/j.livprodsci.2004.11.001
O’Connor, C. E., Jay, N. P., Nicol, A. M., & Beatson, P. R. (1985). Ewe maternal behaviour score and lamb survival. Proceedings of the New Zealand Society of Animal Production, 45 159–162.
O’Connor, C.E., Lawrence, A. B. and Wood-Gush, D. G. M. (1992). Influence of litter size and parity on maternal behaviour at parturition in Scottish Blackface sheep. Applied Animal Behaviour Science 33: 345–355. https://doi.org/10.1016/S0168-1591(05)80071-1
Simm, G., Conington, J., Bishop, S. C., Dwyer, C. M., Pattinson, S. (1996). Genetic selection for extensive conditions. Applied Animal Behaviour Science, 49(1), 47-59.
SMARTER deliverable D2.4. New prototype and report for industry on GPS-generated phenotypes for behavioural adaptation to extensive grazing systems; artificial rearing adaptation phenotypes; lamb vigour scores linked to lamb survival; new foetal and neonatal survival phenotypes (in preparation).
von Borstel, U. K., Moors, E., Schichowski, C., & Gauly, M. (2011). Breed differences in maternal behaviour in relation to lamb (Ovis orientalis aries) productivity. Livestock Science, 137, 42-48. doi:10.1016/j.livsci.2010.09.028
Vouraki, S., Papanikolopoulou, V., Argyriadou, A., Priskas S., Banos, G., Arsenos, G. (2025). Phenotypic and genetic parameters of grazing behaviour of semi-extensively reared Boutsko sheep. Applied Animal Behaviour Science, vol. 282, Jan 2025, 106473. https://doi.org/10.1016/j.applanim.2024.106473
Guidelines on recording lifetime resilience in sheep and goats
Change Summary
| Date of change | Nature of Change |
| October 2024 | First version |
| November 26th 2024 | Comments made by JC |
| December 23rd 2024 | Comments made by MS |
Introduction and scope
Introduction
Lifetime resilience is often tackled through longevity and aspects of productive longevity. Longevity is a trait to quantify productive lifespan of livestock, and for increasing durability and profitability of farms. In dairy ruminants, longevity definitions include: (i) true longevity (all culling reasons, including milk productivity); and (ii) functional longevity (all culling reasons, except voluntary productivity, such as milk productivity or growth). Functional longevity (corrected for production level – milk, growth) reflects the animals’ accumulated ability to overcome health and nutritional challenges. It is an indirect global approach to quantify adaptive capacity to various production environments. Different indicators may be calculated. One indicator is the length of productive life which is computed as the time interval (in days) between first lambing/kidding and culling. Longevity is linked with various predictors, such as fertility, udder health and conformation, resistance to disease, body condition score changes across ewe/doe lifetime. These predictors may be used in breeding program to get an earlier breeding value of longevity and may help to manage and monitor lifetime resilience at the farmer level.
Scope
The scope of these guidelines is to define approaches for the definition of longevity as well as the traits that can be calculated, and the downstream analyses that can be set up (including the use of early predictors to enhance longevity in the evaluation process).
To propose a grid for setting up an observation of the culling causes.
Longevity
Definition, terminology, rationale
The notion of longevity can cover several meanings. Longevity can be understood as the true longevity, i.e. the ability of the animal to live as long as possible, whatever its production level and its functional characteristics. Animal longevity also depends on the replacement rate which is often a choice of the breeders. Animals may be culled due to production level such as milk production or growth or fat/muscle depth, leading to ’voluntary’ culling (i.e. an animal is culled because we want to do it). In contrast, ‘involuntary culling’ is defined as an animal having to leave the flock or herd due to illness / accident/ functional disability etc (i.e. they are culled because we have to do it)
Involuntary culling may be due to:
- Udder health problem (clinical, subclinical, chronic mastitis).
- Lack of resistance to disease such as parasites.
- Problem of footrot.
- Unfavourable shape of the udder (lack of adaptation to machine milking or to suckling).
- Unfavourable general conformation.
- Undesired behaviour (temperament in the milking parlour).
- Infertility or any problem of reproduction.
- Problem of feet or legs, lameness.
- Lack or excess of body tissue mobilisation.
any other undesirable aspect associated with the animal’s inability to produce. Voluntary culling may be due to:
- Low productivity,
- Management decision to cull for age,
- Management decision to cull for a specific coat colour / other phenotype that does not meet the type desired,
- Farmer doesn’t like the animal,
- Economic reason to reduce the number of breeding animals in the flock/herd.
Even if some of these reasons for culling may be considered per se in the selection process by phenotyping and evaluating related traits (for example resistance to mastitis, resistance to gastro- intestinal parasite, fertility, udder morphology), it is often not possible to account for all of them. If properly modelled, functional longevity may be considered as a global and composite approach, allowing to assess the sustainability of the population in selection and of the practiced selection.
For this, different traits may be considered, quite often they are relatively easy to compute with data usually already existing in the genetic database (ex. length of productive life, which can be calculated as the culling date minus the date of the first lambing). There is no additional recording to set up. The difficulties in handling functional longevity are related to the modelling of the trait, given that the trait is fully known when the animal is culled. When not yet culled, the model to set up are quite complex. An example of this was reported by Brotherstone et al. (1997) for dairy cattle and Conington et al. (2004) for hill sheep, whereby live animals’ EBVs for longevity are based on their probability of survival at a given age combined with actual cull dates of relatives that became breeding females in the flock.
Even though there is no need to identify/know the cause of culling, the knowledge of the cause of culling might be a relevant observation of the hierarchy of the culling cause, which may lead to put an emphasis on some specific issue. For example, if we observe an increase in some culling causes (let’s say parasitism) this should lead to a deliberate selection programme to breed more resistant animals to parasites.
One drawback of the functional longevity trait is its lack of precocity. As stated above, it is necessary to have the date of culling or to have accumulated enough lactation to compute the trait. And an appropriate model (e.g. survival analysis) can only partially disentangle this difficulty. It is possible to address this issue by running a multi trait genetic evaluation model combining the longevity trait and some other proxy traits (such as udder morphology, udder health, etc). The use of Genomic Selection is a complementary way to generate early prediction of genetic merit for longevity, provided there is good accuracy of the EBVs of animals in the associated reference population.
Data recording
Longevity traits
The table 1 presents some criteria commonly used in small ruminants to measure longevity. Here, the criteria deal with true longevity, the only one measurable in herd/flocks. Functional longevity will be estimated later, at the statistical analysis step. Table 1 also shows the data required for calculating the longevity criteria. For example, the length of productive life is referred to as the difference between the time a female enters the breeding flock/herd and the date she exits it due to being culled or dying. It is important to notice that the culling date, which is rarely recorded by the farmers, can be replaced by the date of the last event registered for the animal (for example, date of the last performance recording, or of the last reproduction event).
Table 1. Definition of some commonly used longevity criteria
| Longevity criteria | Raw data required | Calculation |
| Length of total lifespan (LTL) | Birth date (BD)
Culling or death date (CD) |
LTL= CD - BD
in days (or months or years) |
| Length of productive life (LPL) | First lambing/kidding date (FKD)
Culling or death date (CD) |
LPL = CD – FKD
in days (or months or years) |
| Total number of days in production (NDL) | Days in milk per lactation (DIM)
or Lambing/kidding date + dry off date for each lactation |
NDL = ∑ DIM |
| Number of lactations (NLACT) | Each lambing/kidding event (KE) | NLACT = ∑ KE |
| Number of lambs or kids during lifetime (NLAMB) | Prolificacy at each lambing/kidding (PR). This may or may not include no. lambs born dead + no. lambs born alive | NLAMB = ∑ PR |
The length of total lifespan can be estimated easily, with only two variables usually registered by farmers. The difference with the length of productive life is that it considers the period when animals had the first lambing/kidding as well as the lambing/kidding interval. If the age at the first lambing/kidding and the lambing/kidding interval are similar between animals, the length of total lifespan will be very close to the length of productive life.
The total number of days in production only covers the “useful” life of the females because it doesn’t include the unproductive periods (such as dry off or large lambing/kidding interval after reproduction failure), compared to length of productive life. But the number of variables necessary to compute it is larger.
For the total number of lambs or kids during a lifetime, it is necessary to include all live-born lambs/kids only or those reared to weaning, if these data are routinely recorded.
Calculation of traits
The last column of Table 1 indicates how to calculate the different longevity criteria, from the raw variables.
The length of total lifespan and the length of productive life are estimated as differences in days between two dates: i) the culling date and ii) the birth date or the first lambing/kidding date, respectively. The total number of days in production corresponds to the sum of the days in milk of each lactation of the female. For the last two criteria (number of lactations or number of lambs/kids), the estimation corresponds to cumulative performance across lifetime.
Instead of waiting for the end of the animal's life to calculate the longevity criterion (which is sometimes long), one solution deals with limiting the animal career to a maximum number of years or lactations. For example, the length of productive life can be calculated only on the first 6 lactations. Subsequently, the length of productive life will be defined as the total number of days between the first lambing/kidding and the end of the 6th lactation. In the same way, the total number of lambs/kids can be estimated at a fixed age, 8 years old for example.
Use for genetic analysis / genetic evaluation.
Models
The genetic ability for longevity is evaluated via the functional longevity, i.e. the true longevity corrected for production traits. Functional longevity is defined at this step, by integrating the level of production as fixed effect in the analysis of longevity criteria described in Table 1.
Different methods are used for the genetic evaluation of longevity traits.
The first method is based on linear models. The main advantage of these models is their ease of implementation because they are used for most of the traits under selection. But they have different drawbacks regarding longevity:
- they do not fit well longevity because longevity indicators do not follow a normal distribution
- they consider only animals that have finished their productive life (unless separate predictors are used). This has two consequences: the longevity data are skewed if living animals are ignored; the breeding value is available lately in the life of the animals. This is notably the case for males for whom most of their offspring must be culled to be evaluated.
- they are not able to include time-dependant variables (e.g. parity, lactation stage). Time dependant variables are useful to take into account the changes in breeding conditions that occur during the life of the animal, and thus to better model longevity data.
The second method is based on proportional hazard model or survival analysis. This type of model counterbalances all the drawbacks of linear models and thus, are the best ones to estimate breeding values for functional longevity. Nevertheless, they are complicated to implement in a routine genetic evaluation process, and a few software exist for genetic survival analyses such as Survival kit, (Ducrocq et al, 2005). However, an evaluation based on an animal model is not feasible in large dataset, leading to use sire-maternal grand-sire models or sire models. Under this assumption, ewes/does EBVs are not available (Ducrocq, 2001).
A third method, less widespread, considers the first three lactations as separate traits in a multiple trait animal linear model. Each lactation is assigned to 1 (instead of 0) once the female reaches the next lactation.
Factors of variation
The main factors of variation of longevity data are:
- herd/flock
- year
- kidding/lambing season
- birth season
- age at first lambing/kidding
- breed
- herd/flock size and herd/flock size variation
- lactation stage, parity (if survival analysis model)
- number of lambs/kids born and reared (for meat sheep and goats)
- within herd/flock production level: this factor of variation is essential to integrate to estimate the functional longevity. Usually, it is the within herd/flock level of production (and not the absolute level of production) that is considered because it explains the decision of the breeder to cull the animal.
Heritabilities of functional longevity
Heritabilities range between 5% and 17% (Sasaki, 2013, Castañeda-Bustos et al., 2014, Geddes et al., 2017, Palhière et al (2018), Buisson et al (2022), Pineda-Quiroga & Ugarte, 2022) indicating that this trait has a low to moderate genetic background. This might be due to the composite signification of longevity, which represents a synthesis of various abilities (see § on predictors).
However, the genetic variation coefficients are moderate suggesting that a genetic variability may be exploited to set up a selection programme.
Genetic correlations
The genetic correlations between functional longevity and other traits are:
- close to 0 for milk production traits. This results from the model, in which longevity is corrected for level of production,
- from 0 to 0.40 for udder type traits (Castañeda-Bustos et al., 2014). The rear udder attachment and the udder floor position are the most correlated to functional longevity,
- from 0.20 to 0.50 for general conformation,
- from 0.01 to 0.15 for reproduction traits (kidding interval, age at first kidding, artificial insemination fertility),
- from -0.15 and -0.40 for somatic cell counts.
EBVs and reliabilities
For dairy animals, because of the low accuracy of breeding values, only males (and especially artificial insemination males) evaluated from the longevity data of their daughters, have EBVs that can be used for selection. A minimum number of daughters culled per sire is required to reach a sufficient accuracy. The consequence is that the AI males get their first longevity EBV quite late in their life. Survival analysis models, because they consider censored data (living daughters), enable better accuracy and thus, an earlier EBV for AI males.
Other strategies are possible to increase the accuracy of functional longevity EBVs:
- introduce genomic information in the genetic evaluation
- use a multiple trait model, including both functional longevity and other traits considered as predictors of longevity listed below.
Given the low heritability of survival traits, the fact that it is expressed late in life (at death or culling), the trait becomes accurate enough when sufficient information on culling or reproduction/lactation is available. It is necessary to enhance direct evaluations by indirect information coming from early predictors. Some relevant predictors are listed below:
- Morphological traits, such as general conformation or udder morphology (especially in dairy species),
- Reproduction traits (fertility, lambing/kidding interval, age at first lambing/kidding, pregnancy scan results, …),
- Udder health, and particularly milk somatic cell count,
- Resistance to disease such as resistance to parasites or to footrot,
- Traits related to feet and legs, such as lameness or twisted or bowed legs, closed or opened hocks,
- Serum immunoglobulin concentration in the early life (Ithurbide et al, 2022a),
- Maturity (dairy species) that can be defined as the ability to maintain a good level of production over the parities, independently of the level of production on the whole lifetime (equivalent of a persistency, but over the lactations and not over the test-days) (Arnal et al, 2022),
- Milk metabolites (Ithurbide et al, 2022b)
- Body tissue mobilisation (McLaren et al., 2023). It was demonstrated that ewe tissue mobilisation was genetically associated with ewe fertility and productive longevity (such as pregnancy scan result, foetal loss from scan to lambing, lamb loss from lambing to weaning, number of lambs weaned). It is made possible by collecting body condition score (BCS) data throughout the reproductive cycle (e.g. pre-mating, pregnancy scan, pre lambing, mid lactation, weaning) and calculating gain or loss of BCS between physiological stage.
These predictors are linked to longevity traits. An unfavourable udder shape, reproduction disorders, a susceptibility to a given disease or a low maturity may lead to involuntary culling and therefore a low longevity of the animal. Few genetic correlations have been published but correlations between EBVs show favourable correlations between these predictors and longevity.
Longevity traits, once evaluated, either in linear or survival analysis model, may be combined with the longevity traits in a multi-trait evaluation, to incorporate the information from early predictors.
A full multiple trait evaluation is not feasible in large datasets. Therefore, approximate strategies must be used, such as considering records adjusted for all non-genetic effects in linear models (yield deviation or daughter yield deviation, other type of pseudo records), or sub-indices incorporating traits that are linked together e.g. pulling together data on footrot, mastitis and parasite resistance could be considered together in a ‘health’ sub-index.
Culling causes
Even though the knowledge of the causes of culling is not necessary to generate a phenotype of longevity and an EBV of functional longevity, the knowledge of the causes of culling, through an observation based on a sufficient panel of flocks/herds, and repeated each year, may give relevant information on the hierarchy and the evolution of the culling causes. It may also enable better understanding of the strategies of culling by farmers leading to better modelling of functional longevity.
Culling causes may be collected with different levels of precision, from a general group of causes to a precise cause, through intermediate information.
In sheep as in goat, the following group of culling causes may be collected:
- Udder health (mastitis)
- Udder morphology
- Production ability
- Respiratory disorders
- Reproduction disorders
- Digestive disorders
- Nervous disorders
- Musculoskeletal disorders
- Skin disorders
- Conformation
- General condition
- Age
- Behaviour
- Accident
- Other ailments (e.g. sudden death, brucellosis, intoxication, fever …)
- Voluntary culling
Each group may be completed with sub-group or precise cause. Below are two examples, first for udder health (table 2), second for reproduction disorders (table 3).
Table 2. Detailed categorisation of udder health culling causes
| Group | Sub-group | Specific cause |
| Udder health (mastitis) | Gangrenous mastitis | Gangrenous mastitis |
| Brief mastitis | ||
| Characteristic symptoms | Mastitis | |
| Clinical mastitis | ||
| Mastitis during suckling | ||
| Coliform mastitis | ||
| Listeria mastitis | ||
| Mastitis before lambing/kidding | ||
| Agalactia mastitis | ||
| Functional symptoms | Blood in the milk | |
| Chronic mastitis, palpation | induration of the udder | |
| Bumps in the udder | ||
| Nodules | ||
| Mammary abcess | ||
| Saggy udder | ||
| Visna mastitis | ||
| Unbalanced udder | Milk in one side | |
| Unbalanced udder | ||
| Subclinical | Subclinical mastitis | |
| Somatic cell count (SCC) and California mastitis test– CMT | ||
| Other | Other |
Table 3. Detailed categorisation of reproduction disorders culling causes
| Group | Sub-group | Specific cause |
| Reproduction disorders | Fecundity | Open + infertile |
| Lately fertile, out of season | ||
| Ram infertile | ||
| Gestation | Abortion | |
| Vagina or rectal prolapse | ||
| Pregnancy toxaemia | ||
| Difficult gestation | ||
| Early abortion | ||
| Late abortion | ||
| Lambing/kidding | Difficult lambing/kidding | |
| Caesarean | ||
| Uterus inversion | ||
| Infection during lambing/kidding | ||
| Vagina or rectal prolapse | ||
| non deliverance | ||
| Acute metritis | ||
| Chronic metritis | ||
| Miscellaneous | Reproduction disorders | |
| Vaginal sponge infection | ||
| Hermaphrodite | ||
| Various | ||
| Male: testicles | 1 testicle | |
| Small testicles | ||
| Abscess | ||
| Contagious epididymitis | ||
| Male: penis | Urinary gravel | |
| Wound | ||
| Phimosis |
Acknowledgements
We gratefully acknowledge the contributions to these lifetime resilience guidelines by the following people:
- Joanne Conington, SRUC, the UK
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
- Isabelle Palhière, INRAE, France
- Jean-Michel Astruc, IDELE, France
- Carolina Pineda Quiroga, NEIKER, France
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Arnal M., Palhiere I., Clément V. (2022). Maturity, a new indicator to improve longevity of Saanen dairy goats in France. Proceedings of 12th World Congress on Genetics Applied to Livestock Production (WCGALP), Jul 2022, Rotterdam, Netherlands. doi:10.3920/978-90-8686-940-4_738.
Brotherstone, S., Veerkamp, R. F. and Hill, W. G. (1997). Genetic parameters for a simple predictor of the lifespan of Holstein-Friesian dairy cattle and its relationship to production. Animal Science 65: 31-37.
Buisson D., J.M. Astruc, L. Doutre, I. Palhière. Toward a genetic evaluation for functional longevity in French dairy sheep breeds. Proc 12th WCGALP, 2022
Castañeda-Bustos, V. J., Montaldo, H. H., Torres-Hernández, G., Pérez-Elizalde, S., Valencia-Posadas, M., Hernández-Mendo, O., & Shepard, L. (2014). Estimation of genetic parameters for productive life, reproduction, and milk-production traits in US dairy goats. Journal of Dairy Science, 97(4), 2462-2473.
Castañeda-Bustos, V. J., Montaldo, H. H., Valencia-Posadas, M., Shepard, L., Pérez-Elizalde, S., Hernández-Mendo, O., & Torres-Hernández, G. (2017). Linear and nonlinear genetic relationships between type traits and productive life in US dairy goats. Journal of Dairy Science, 100(2), 1232-1245.
Conington, J., Bishop, S. C., Grundy, B., Waterhouse, A., & Simm, G. (2001). Multi-trait selection indexes for sustainable UK hill sheep production. Animal Science, 73(3), 413-423.
Conington, J., Bishop, S.C., Waterhouse, A. and Simm, G. (2004). A bio-economic approach to derive economic values for pasture-based sheep genetic improvement programmes. Journal of Animal Science 82: 1290-1304. https://doi.org/10.2527/2004.8251290x
Ducrocq, V. (2001). A Two-Step Procedure to get Animal Model Solutions in Weibull Survival Models Used for Genetic Evaluations on Length of Productive Life. Interbull Bulletin, vol.27, pp.147-152
Ducrocq, V. (2005). An Improved model for the French genetic evaluation of dairy bulls on length of productive life of their daughters. Animal Science, 80(3), 249-256.
Geddes, L., Desire, S., Mucha, S., Coffey, M., Mrode, R. and Conington, J. (2018). Genetic parameters for longevity traits in UK dairy goats. IN: Proceedings of the World Congress on Genetics Applied to Livestock Production, Volume Species - Caprine: 547.
Ithurbide, M., Huau, C., Palhière, I., Fassier, T., Friggens, N. C., & Rupp, R. (2022a). Selection on functional longevity in a commercial population of dairy goats translates into significant differences in longevity in a common farm environment. Journal of Dairy Science, 105(5), 4289-4300.
Ithurbide, M., Wang, H., Huau, C., Palhière, I., Fassier, T., Pires, J. & Rupp, R. (2022b). Milk metabolite profiles in goats selected for longevity support link between resource allocation and resilience. In Proceedings of 12th World Congress on Genetics Applied to Livestock Production (WCGALP) pp. 276-279
McLaren A, Lambe, N R and Conington J. (2023). Genetic associations of ewe body condition score and lamb rearing performance in extensively managed sheep. 105336. Livestock Science September 2023 https://doi.org/10.1016/j.livsci.2023.105336
Palhière I., C. Oget, R. Rupp, Functional longevity is heritable and controlled by a major gene in French dairy goats, 11th WCGALP, Auckland, Nouvelle-Zelande, 11-16 février 2018
Pineda-Quiroga, C., Ugarte, E. (2022). An approach to functional longevity in Latxa dairy sheep. Livestock Science 263, 105003
Sasaki, O, (2013), Estimation of genetic parameters far longevity traits in dairy cattle: A review with focus o n the characteristics of analytical models, Animai Science Journal, 84(6), 449-460,
SMARTER Deliverable 2,2 - "New breeding goals far lifetime resilience far materna!sheep breeding programmes"
Recording of the environment
This section tackles the record of the environment addressed by the ICAR guidelines. For more clarity, the record of the environment is described in a specific document that has its own template and is consistent by itself. The description of this sub-section may be updated when needed.
Guidelines on recording the environment in sheep and goats
Change Summary
| Date of change | Nature of Change |
| October 2024 | First version |
Introduction and scope
Introduction
In the genetic evaluation process, the genetic model includes environmental effects (generally fixed effects, in some cases random effects) to correct the phenotypes from these effects, not related to the genetic value of the animal. These environmental effects that affects the expression of the genotypes depend on the traits and the method of phenotyping, the environment itself (flock/herd, year, parity, season of lambing, number of born or reared lambs/kids, scorer, gender of the lamb/kid, management of mob groups, etc). The quality of the record of the environment is important to correct relevantly the performance of the animal.
Some other environmental effects that are usually included in a general flock/year or management mob group effect could be identified, such as the feeding effect or the climate effect. By including these effects in the genetic model, we could get less biased and more precise EBVs, especially when these effects are individualised or are period-specific (feeding might depend on such and such groups of animals, climate might influence the performance of such and such test- day). Moreover, the more precise knowledge of environmental effect might be valorised for flock/herd management and extension services towards farmers.
Moreover, feeding can be considered as an environmental effect, but as well be constitutive of a performance. This is typically the case for feed efficiency where the quantity and the quality of the diets allows to calculate the phenotype.
Likewise, with the climatic change, breeding for animals more resistant or more resilient to higher temperatures (especially thermal stress) becomes a selection objective per se (example of heat tolerance). In this context, the conditions of temperatures (or temperature/humidity combination) not only might be an environmental factor, but be part of the phenotype.
Other environmental effects can be described and should enrich this document in the future.
Scope
This document focuses on those data that are worth recording the precise the environment or to calculate novel traits of interest.
Following SMARTER work, the document will describe the record of the diet (section 3) and the record of meteorological data (section 4)
Further factors might be described later, letting this document open to new section in the future, including:
- Recording the diet in small ruminant
- Recording meteorological data
- Other environmental records
Recording the diet
Definition, terminology, rationale
Recording the diet consists in collecting data on the quantity and quality of a ration that an animal, a group of animals of a flock/herd consumes at a given period.
The characterisation of the ration, in terms of energy and protein depends upon the countries. For example, the French INRAE Feeding System for Ruminants (Nozière et al., 2018) is different from the British one (AFRC, 1993). This is the reason for which we will describe in this section general recommendations, that can be applied, translated to the domestic feeding system used
Breeding for more efficient animals is more and more important for economic reason (the feeding resources are costly, might be rare in years with climatic excess such as heat or drought) and for environmental reasons (feed/food competition, emission of green-house gases). Feed efficiency is a trait of high interest in this context. Even though it is deceptive to calculate gold standard efficiency trait in private farm, the knowledge of diets in those farms should help to correctly manage the proxies that are promoted in SMARTER. Diet could also be used as a corrective factor in evaluation models in the future. In addition, it might be a support to better understand the herd/flock effect and its variation across year, and therefore give more acute and relevant advice to the farmers.
It is difficult and time-consuming to collect the data for establishing the diet in the flock/herds. The diet is collective in most of the situations (the same amount of forage is given to all animal because the forage is not given individually). When the concentrate is given through Automated Concentrate Feeder (ACF) in the milking parlour, the individualisation is not at the animal scale but at a limited number of groups scale. That’s why we suggest recommendations that must be adapted to each situation.
The aim is to tend to the better possible estimation of the forage ingestion, given that the direct measurement is impossible in commercial farms. Proxies are studied to get indirect measurement of the intake, but they are not validated so far (Near Infra Red Spectra technique). As soon as validated results are available, these recommendations will be updated.
Data recording
When to record the diet
The diet may be recorded at relevant period of the physiological status of the animals in the flock/herd. It is possible to take advantage of the visit of a technician to record the ration (for example when performance recording such as at each (or some of the) test-day when milk recording, or at weighing visit in meat sheep performance recording.
Below are examples of relevant physiological status:
- At mating (or before the mating and after the mating)
- End of gestation (in the month preceding the lambing/kidding)
- After lambing/kidding
- At weaning or just after weaning (peak of production in dairy animals)
- Dairy animals: at each test-day or at some of the test-day
In case of ACF (Automatic Concentrate Feeder), it is possible to record the distribution of concentrate more frequently.
It may be useful to establish the requirements of animals (on average) at each point of diet record. The requirements must concern the energy (in the unit usually used in the country) and the protein (in the unit usually used in the country).
How to record the diet
Individual diet
- This can be obtained through ACF for concentrate, mainly in the milking parlour.
- Intake of forage cannot be collected individually but can be predicted through the intake capacity system, such as the one proposed by INRAE (Nozière et al., 2018).
Collective diet (at the flock/herd scale or at the mob scale)
- Forage (hay, or haylage): some bales of each preservation technic can be weighed once a year with a dry matter (DM) measurement for haylage (it can substantially vary). For hay, DM can be estimated at 85%. Afterward, we can just record how many bales of a given quality (several cutting stages are preserved and not given at random) are distributed per flock per time unit. For silages, it is more complicated, but based on the same procedure, we can weigh one distribution (assuming that it will be constant over time) and simultaneously measured DM. In both situations, if refusals cannot be measured, they must be sufficient for assuming an ad libitum distribution. When the feeding system used in the country can predict the DM intake through the intake capacity of the animal and the quality of the feed, individual diet can be estimated.
- Grazing: for dairy sheep grazing within a short duration per day or the full day, intake can be estimated through ad hoc system. As an example, the new French INRATion feeding software (INRATion V5®) proposes such estimation based on grazing duration, biomass availability and quality.
Defining the constitution of the diet
Precise the type of distribution of the ration
- collective ration
- individual ration (concentrate when ACF)
- pasture
Categories of feedstuff
- Hay
- Partially or fully fermented fodder and fodder preserved by silaging or wrapping:
- Silage
- Wrapped bales
- Pasture
- Straw
- Green feeding
- Dehydrated alfalfa
- Pulp (dehydrated beet pulp, citrus pulp, etc)
- Cake (soybean, rapeseed or sunflower seed)
- Cereals grain (wheat, barley, maize, etc)
- Complete commercial concentrate
- Other by-products of agro-food industry (cereal brans, brewer’s grains, hulls etc.)
Species
For each category, specify the species (rye grass, alfalfa, clover, maize, wheat, barley, etc), physiological stage or age of regrowth, and harvest conditions (cutting length of the forage and added preservative or not for silages, conditions of hay making drying in the field or mechanically dried).
Characterizing the diet
Quantity
Quantity distributed, refused, consumed. Check that these amounts are regularly distributed, refused and consumed because it can markedly influence the animal performance specifically for dairy animals at test day.
The quantity of each feedstuff may be expressed in kg dry matter for forage, in kg gross matter for concentrate. However, final diet for requirement calculation must be expressed as DM.
Requirements
Requirements for the main categories of animals: it depends on the physiological status (maintenance, production, growing, pregnancy)
Average requirement coverage ratio (energy and nitrogen). For example, the requirement coverage ratio in French dairy sheep is roughly 115% for energy and about 125% for nitrogen of the requirements of the average ewe. That allows covering the requirements of about 85-90% of the flock. Difference between energy and nitrogen is assumed to be covered through the body reserve mobilisation.
Quality characterization
The feedstuffs and the ration must be characterized at least in terms of
- Energy
- Protein (or nitrogen)
In case of commercial concentrate, data written on the label are used.
Energy and protein can be expressed in the current unit used in the country. For example, in France, energy is expressed in UFL which is equal to 1.7 Mcal Net energy (Nozière et al., 2018).
It may also be expressed in the international unit, which can be Mcal or MJ.
Use for genetic analysis / genetic evaluation
Diet as part of a phenotype
Calculation of feed efficiency phenotypes: see recommendations on feed efficiency.
Diet as part of a factor in the evaluation model
In most of situations it is impossible in small ruminants to establish individual consumption, for practical reason. The collective effect of the diet is explained in the flock/year effect. The intermediate situation should be when ACF allows to identify several groups within the flock/herd, at a specific test-day or visit. It is possible in this case to put in the model a mob effect grouping animals being given the same amount of concentrate. This should result in a more precise calculation of the breeding value of the animal. Nevertheless, this approach has so far not be used to our knowledge.
Meteorological data
Definition, terminology, rationale
Meteorological conditions may affect the environment effect on the traits of interest. Even though they may be absorbed in a flock effect at the scale of the year or at the scale of a given test-day, it is relevant to be able to quantify the effect of such and such meteorological parameter (and especially the heat stress) ot the zootechnical traits. The global warming and the higher temperature in which the animals are bred emphasises this interest. It is possible to better assess the comfort zone of the populations, that means the meteorological conditions in which the zootechnical traits are not affected. It is also possible to identify animals better adapted to an increase in temperatures or able to be resilient to a wide range of temperatures, that means to maintain their productive ability. In this case, meteorological data, combined with a production trait (growth, milk production, milk composition) or fertility trait, are used as a resilience characterisation by assessing the ability of the animals to recover their production following meteorological challenges.
Meteorological data are mostly temperature, humidity, precipitations, wind speed and radiations. An issue in small ruminants is to select for adapted animals to new environmental challenges, without artificializing their environment of breeding. Mainly because the economic and societal constraints are such as breeding animals outdoors on pasture is desired and breeding indoors inartificialized environment may be costly in terms of energy.
Data recording
Meteorological data from weather station
The aim is to affect outdoors meteorological data to a farm. This can be obtained by assigning to the farm the meteorological data of the closest or more relevant weather stations, using the geographical coordinates of both the farm and the weather station.
The following data may be used:
- Temperature (minimum, maximum, average)
- Relative humidity (amount of moisture in air compared to the maximum amount of moisture it can have at a specific temperature). Expressed in %.
- Specific humidity (ratio of water vapor mass to the total mass of air and water vapor.
- Wind speed
- Precipitations and precipitation type
- Solar radiation
- Atmospheric radiation
- Evapotranspiration
Different index accounting for weather factors have been proposed. One of the most popular is the Temperature Humidity Index (THI) which may be calculated to get a single value representing the combined effects of air temperature and humidity associated with the level of thermal stress.
Different formulas of THI are proposed in the literature. Below is an example of formula proposed by Finocchiaro (et al., 2005):
THI = T − [0.55 × (1 − RH)/100] × (T − 14.4)
where T is the mean daily in °C and RH is the mean relative humidity expressed in percent. Quite often, the parameter used in the analysis model is the temperature of the THI (mainly because temperature and relative humidity are the most available parameters).
Let us also mention the Heat Load Index, referred to as the 'HLI', which is an index that brings together all the weather factors into one number to allow easy interpretation of the cooling capacity of the environment.
The assignation of meteorological data to a farm depends on the countries and on the availability of weather data.
In some countries, the territory may be cut out in a grid, each cell of the grid being considered to have the same meteorological parameters because they are close to the same weather station of reference. As an example, this is the case in France with a grid named SAFRAN cutting the territory into 9892 cells of 64 square kilometres each [8 km by 8 km] (Annex 1). This grid was used, thanks to specific permission from Meteo France, to affect each farm of a given project (by using its GPS coordinate) to a single cell of the grid and thus get relevant meteorological parameters.
The meteorological spatialised data are collected from weather station, on which specific interpolation are applied to present these data on the SAFRAN grid.
The meteorological data key period to consider must be thought according to the production system associated to the breed, type of traits measured and analysed. For example, for milk production (milk recording), we may consider the 3 days preceding the test-day. For semen production, we may consider the meteorological data either at the day of the semen collection, or during the spermatogenesis, which is around 50 days before the semen collection. For the insemination itself (which is in case of fresh semen the same day as semen production), we may consider climate data either the very day of the insemination operation or during a week preceding it.
Environmental data from sensor in the farm
Temperature and humidity may also be collected on site, thanks to sensors situated on-farm, for example in the sheep pen or the stable.
The number of sensors may depend upon the situation and configuration of each building, the goal being to be representative of the pen. In the practical situations of the SMARTER project, 2 to 3 sensors were set in the pen where animals are indoors at a height of 2 meters above the ground, so that they are protected from the animals. If the pen is already equipped by sensors, it is possible to retrieve the data from the existing sensors. The sensors must cover all the relevant groups of animals (primiparous, multiparous, etc), even if they are in different buildings. Measures might be collected several times a day, for example once an hour, to get a precise evaluation of the daily temperature and hygrometry. To relevantly collect the atmosphere of the building, the sensors must be set in a place free from too much air flow or too much sunshine. It is important to regularly check the batteries to avoid loss of data.
Use for genetic analysis / genetic evaluation
Effect of meteorological parameters (eg. temperature or THI) may be estimated on zootechnical traits, using different types of linear models.
The parameter may be considered as a categorical variable (each degree of the parameter being defined as a different class). Or it may be considered in a linear regression on degrees of the parameter.
Reaction norms model, using Legendre polynomial for example, may be used to assess populational losses of the zootechnical trait due to high or low temperature and/or humidity.
Two types of analysis can be made:
- a populational analysis (populational response to the effect of temperature or THI). It gives the comfort rage of each population and how much the loss is with lower or higher temperature or THI.
- an analysis of the genetic components using a random regression model. It permits to estimates genetic parameters of traits according to the temperature or THI and to calculate EBVs of animals at different temperatures or THI levels. Such EBVs allow to identify less vulnerable animals along a range of climate values, so as to identify and select the most robust animals.
Other environmental record
To be completed (or not) when necessary
Acknowledgements
We gratefully acknowledge the contributions to these environment documentation guidelines by the following people:
- Jean-Michel Astruc, IDELE, France
- Antonello Carta, Agris, Italy
- Philippe Hassoun, INRAE, France,
- Gilles Lagriffoul, IDELE, France
- Carolina Pineda Quiroga, NEIKER, Spain
- Eva Ugarte, NEIKER, Spain
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Finocchiaro R, van Kaam JB, Portolano B, Misztal I. Effect of heat stress on production of Mediterranean dairy sheep. J Dairy Sci. 2005 May;88(5):1855-64. doi: 10.3168/jds.S0022-0302(05)72860-5. PMID:15829679.
Nozière, P., Sauvant, D., Delaby, L. 2018. INRA Feeding System for Ruminants. Wageningen Academic Publishers, 640 p., 2018, 978-90-8686-292-4. ⟨10.3920/978-90-8686-292-4⟩. ⟨hal-02791719⟩
AFRC (Agricultural and Food Research Council). 1993. Energy and protein requirements of ruminants. CAB International, Wallingford.
Annexes
Acknowledgements
We gratefully acknowledge the contributions to these guidelines on recording resilience-related traits and the environment in sheep and goat by all the people working in the ICAR working group on sheep, goat, camelids and/or participating to the SMARTER project:
The different documents giving the recommendations of each sub-sections list in their own acknowledgements the persons involved in the writing of the guidelines.
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Guidelines on resilience traits in sheep and goats
| Date of change | Nature of Change |
| October 2024 | First version |
| November 2024 | Tracked change revisions by JC |
| December 2024 | Tracked change revisions by MS |
Introduction and scope
Introduction
Genetic selection including behavioural traits could be an advantageous strategy for improving robustness and welfare of farm animals in various farming conditions by minimizing unsuitable responses to changes in their social and physical environment, limiting an excessive fear of humans and improving sociability (Mignon-Grasteau et al., 2005). Farm animals are social and gregarious, and relational behaviours are essential for ensuring social cohesion, social facilitation, offspring survival and docility toward humans. Breed differences and genetic variation within breed have been reported in lambs for early social behaviours and found to be heritable, and associated with some QTL, suggesting such behaviours could be selected early (Boissy et al., 2005; Beausoleil et al., 2012; Hazard et al., 2014; Cloete et al., 2020). In addition, such early social reactivity of lambs towards conspecifics or humans was identified as a robust trait and that selection for early social reactivity of lambs towards conspecifics or humans is feasible (Hazard et al., 2016; 2022).
The behaviour of both ewes and lambs, and their interaction at lambing, have been widely described. Such behaviour is important for the survival of the offspring, especially in extensive farming conditions as reviewed by Dwyer et al. (2014). Moreover, it has been shown that primiparous ewes are more prone to abandon their lambs due to their lack of maternal experience (Dwyer, 2008) and that lamb survival at birth is lowly heritable (Brien et al., 2014). Taken together these factors could hinder the development of extensive farming systems. Genetic selection on maternal attachment traits could therefore be advantageous to improve offspring survival and growth, and reduce labour, as suggested by Mignon-Grasteau et al. (2005). Genetic variations in maternal behaviour between breeds of sheep have been well documented (for review see: Dwyer, 2008; von Borstel et al., 2011) while little was known about within-breed genetic variability and even less about maternal reactivity traits. We hypothesized that maternal attachment to the litter has a genetic component in sheep, and we recently reported that as expected the maternal reactivity at lambing is a heritable trait (Hazard et al., 2020;2021).
Grazing behaviour is also important for animals raised in extensive production systems because it can support adaptability to changing environments. In particular, small ruminants reared in semi-extensive systems face many environmental and welfare challenges that are difficult to quantify. The evidence in the literature suggests that there are differences in grazing behaviour between and within breeds of sheep (Simm et al., 1996; Brand, 2000). The notion is that natural selection combined with subjective artificial selection have led to some animals being more adaptive to extensive conditions. In this regard, genetic variation may exist for key grazing behaviour traits (Simm et al., 1996; Dwyer et al., 2005), but relevant literature is scarce. During the SMARTER H2020 project, a study was performed on grazing behaviour of the indigenous Boutsko Greek mountainous sheep breed, which is reared semi-extensively. The results showed that duration of grazing and speed are heritable traits (Vouraki et al., 2025).
Acronyms used in these guidelines
- AT Arena Test
- CT Corridor Test
- GPS Global Positioning System
- LS Lambing Site
- PCA Principal Component Analysis
Scope
The aim of the present report is i) to define the behavioural traits of interest, ii) to describe approaches for behavioural measurements, iii) to describe their use for genetic analysis and evaluation.
To-date, the present guidelines describe 3 groups of traits related to behaviour:
- Behavioural reactivity towards conspecifics or humans
- Maternal reactivity
- Behaviour at grazing
Kid/lamb vigour is a relevant behavioural trait, but this trait is tackled within the section “foetus and young survival in sheep and goats” of the guidelines.
Most of the work undertaken on behaviour concerned sheep. This has been particularly the case in SMARTER. Most of the recommendations might be applied to goats as well. Nevertheless, we will use the ovine terms in the guidelines below.

Behavioural reactivity towards conspecifics or humans
Definition, terminology, rationale
Behavioural reactivity towards conspecifics (i.e. sociability):
It is the social motivation of the lambs to join their conspecifics in response to social isolation with or without presence of a motionless human. Expression of higher levels of a panel of behaviours, including vocalisations and locomotion, is hypothesised as an active way to maintain social link with conspecifics.
Behavioural reactivity towards humans (i.e. docility):
It is the reactivity of isolated lambs to a walking human. Higher flight distance between the lamb and a human indicates a lower docility toward a human.
Behavioural reactivity towards conspecifics and humans are measured in standardised behavioural tests (arena and corridor tests, described below).
Higher sociability and/or docility towards humans may improve adaptation of sheep to harsh environments through social facilitation (i.e. transmission of feeding preferences…), social cohesion (i.e. transhumance…) and reactivity to handling. Consequently, improving such behavioural traits may improve welfare, production, and labour of shepherd.
Data recording
Behavioural tests
The test described below have been implemented in France. It must be considered as a possible test, as others can be described later and enrich these guidelines.
Lambs must be individually exposed just after weaning (i.e. approximately 10 days after weaning) to two behavioural tests. The delay between weaning and behavioural tests must be sufficient for the change of social preferences of lambs for their dam to conspecifics.
The arena test (AT) consists of two successive phases evaluating 1) reactivity to social isolation (AT1), 2) the motivation of the lamb towards conspecifics in presence of a motionless human (AT2). The arena test is performed indoors. The arena test pen consists in an unfamiliar enclosure virtually divided into 7 zones as described in detail by Ligout et al. (2011) (Figure 1). On one side of the enclosure (i.e. at the opposite of the entrance), a grid separates the tested lamb from another smaller pen containing 3 or 4 conspecifics. The first phase of the test (arena test phase 1, AT1) starts once the tested animal joins its flock-mates located behind a grid at the opposite side of the arena (time duration for joining: lower than 15 sec). No behavioural recording is performed during the joining. At this time, an opaque panel is pulled down (from the outside of the pen) between the flock-mates and the tested lamb to prevent visual contact. After one minute the phase 1 stops and the panel is pulled up so the lamb can see its flock-mates again. Once the lamb has returned near to its flock-mates, or after 1 minute if the lamb did not do so, a non-familiar human slowly enters the arena through a door located near the pen of the flock-mates and stood 20 cm in front of the grid separating the arena from the lamb’s flock-mates. The second phase (arena test phase 2, AT2) starts once the human is in place and lasts for a further 1 minute.

The corridor test (CT) consists of two successive phases evaluating 1) reactivity to social isolation (CT1) and 2) reactivity to an approaching human (CT2). The test pen consists in a closed, wide rectangular circuit and has been described in detail by Boissy et al. (Boissy et al., 2005) (Figure 2). The first phase (corridor test phase 1, CT1) starts when the lamb enters the testing pen and lasts for 30 seconds. After that time a non-familiar human enters the testing pen and the second phase (corridor test phase 2, CT2) starts and lasts 1 minute. During this phase, the human walks at a regular speed through the corridor (the corridor is divided into 6 virtual zones and one zone is crossed every 5 seconds) until two complete tours has been achieved.
Behavioural traits
Several behaviours are measured during behavioural tests: vocalisations (i.e. frequency of high- pitched bleats), locomotion (i.e. number of virtual zones crossed), the proximity score (i.e. weighting of time spent in virtual zones, a high score indicated a high duration spent close to conspecifics and a human).
An investigator counts the lamb’s vocalisations directly during the tests, from outside the pen using a laptop: number of times the animal bleats with an open mouth (high bleats, AT1/2- HBLEAT, CT1-HBLEAT). Locomotor activity is assessed by measuring the number of virtual zones crossed during arena test phases 1 and 2 (AT1/2-LOCOM) and corridor test phase 1 (CT1- LOCOM). This behaviour can be assessed using video recording or using infrared cells regularly positioned along the AT to detect displacement. The proximity to flock-mates and the human during AT2 is calculated by weighting of time spent in virtual zones (i.e. a high score indicated a high duration spent close to conspecifics and a human).
During CT2, every five seconds throughout this phase, an investigator records with a laptop the zones in which the human and the animal are located. In addition, the walking human records with a stopwatch the total duration during which the head of the lamb is visible. The mean flight distance (DIST) separating the human and the lamb (i.e. knowing the length of each virtual zone) and the time during which the human sees the lamb (SEEN) is measured in CT2.
Calculation of traits
Deviations from normality of row data must be tested using relevant statistical tests (e.g. the Kolmogorov–Smirnov test). Several raw measures must be transformed in order to minimise major deviations from the normal distribution. Square root transformation is applied to AT1/2- HBLEAT, CT1-HBLEAT. A multivariate analysis may be performed to take into account the multidimensional aspect of behavioural responses. Results of principal component analysis (PCA) indicate that the main principal components is structured mainly with similar behaviour (i.e. higher weight of similar behaviours for the different tests on the same component). Consequently, three synthetic variables may be constructed using PCA. Each PCA is performed for a set of similar behavioural variables across the behavioural tests. The first component of each PCA, explaining the largest part of total variance, is defined as a synthetic variable. Two synthetic variables are specific to the reactivity to social isolation: high bleats (HBLEAT, using AT1/2-HBLEAT and CT1- HBLEAT), locomotion (LOCOM, using AT1/2-LOCOM and CT1-LOCOM). One synthetic variable is specific to the reactivity to an approaching human: the tolerance to being approached when the lamb is free to flee (HUMAPPRO, using CT2-DIST and CT2-SEEN).
Use for genetic analysis and genetic evaluation
Genetic analyses and genetic evaluation can be performed on single traits and synthetic variables. Genetic analyses (estimation of (co)variance components and prediction of breeding values) for quantitative behavioural traits may be implemented with a mixed model methodology in animal model. Random effects should include:
- a direct additive genetic effect of the animal (i.e. lamb),
- a maternal permanent environment effect (i.e dam), that describes lamb phenotypic variation caused by the environment of the ewe
- a litter permanent environment effect, that accounts for phenotypic variation caused by the environment of the litter of the lamb being tested.
All relevant fixed effects and interactions should be included in the model. Factors that could be considered include:
- a combination of the litter size at lambing and the number of lambs suckled with their dam
- sex, age, live weight of the lamb,
- dam parity and/or age of dam nested withing parity if needed contemporary group (e.g., depending on the data collection: flock-year-season, grazing location…)
Maternal reactivity
Definition, terminology, rationale
- Behavioural reactivity at lambing (i.e. maternal reactivity). It is the social motivation or attachment of the ewe for the litter expressed in response to an approaching human, or the withdrawal of the litter with or without presence of a human. Expression of higher levels of a panel of behaviours, including maternal behaviour scores, vocalisations and locomotion, is hypothesised as an active way to maintain social link with lambs.
Maternal reactivity is measured in standardised behavioural tests (a scoring test outdoors, an arena test indoors, described in the controlled test below) or a maternal behaviour score (MBS) designed for use in extensive sheep systems as described by O’Connor et al (1985), the genetic basis of which was reported by Lambe et al., 2001 for Scottish Blackface sheep.
Higher maternal reactivity may improve adaptation of sheep to harsh environments through a higher behavioural autonomy at lambing and a reducing dependency to the support provided by shepherds. Consequently, improving such behavioural traits may improve welfare, production, and labour of the shepherd.
Data recording
Behavioural tests
The controlled test described below have been implemented in France. It must be considered as a possible test, as others can be described later and enrich these guidelines. Ewes are individually exposed to two behavioural tests: a scoring test performed just after lambing, outside at the lambing site, and then an arena test performed indoor, one day after lambing. The second test is performed after the bonding period needed to establish the social link between ewes and lambs and which occurs generally within the first twelve hours after lambing (Keller et al, 2003).
Scoring test at lambing site: Maternal reactivity is assessed outside at the lambing site approximately 2 hours after lambing, only on ewes that lambed during daylight when the shepherd approaches the lambing ewes to catch lambs for weighing and identification. Scoring at lambing is not performed in the following situations: if the location of the lambing site does not readily facilitate the testing procedure, if there are perturbations of scoring due to interference by other ewes, for sanitary reasons that could affect behaviours (including difficult lambing, death of all lambs of a litter). Measurement of maternal reactivity at the lambing site (LS) consists of two successive phases: (1) when the shepherd approaches the lambs; and (2) the capture and displacement of the lambs by the shepherd. In the first phase (LS1), the shepherd stands approximately 15 meters away from the lambing spot and approaches the ewes and the lambs at a regular speed (1 m/s). In the second phase (LS2), the shepherd catches all the lambs at the same time and moves away from the lambing spot in the same direction as that of the approach, stopping at the starting point where he places the lambs back on the ground and then moves 15 meters away to allow the ewe to restore contact with her lambs. This second phase of the test is not applied to ewes that flee at the approach of the shepherd and do not return within 60 seconds after the end of LS1.
Arena test: After lambing, all the ewes and lambs (both day and night births) are transferred to a shelter close to the place of lambing and penned individually for few hours. They are then moved to a collective pen until the next day when they are tested in the arena test (24h ± 6h after lambing). The arena test (AT) is performed indoors and adapted from the original test developed by Boissy and colleagues (2005) to investigate social attachment in sheep (Ligout et al., 2011). In the present study, the test consists of three successive phases evaluating the ewe’s 1) attraction to her litter, 2) reactivity to social separation from her litter, and 3) reactivity to a conflict between social attraction to her litter and avoidance of a motionless human. The test pen consists of an unfamiliar enclosure virtually divided into 7 zones (zone 7 being the zone nearest to the litter). On one side of the enclosure, a grid separates the tested ewe from another smaller pen containing her lamb(s). The first phase of the test (AT1) starts when the tested ewe enters the arena and lasts for 30 s. Then, a remotely controlled opaque panel is pulled down in front of the grid to prevent visual contact between the tested ewe and her lambs. The second phase (AT2), during which the tested ewe is separated from her lambs, lasts 1 min. Finally, the panel is raised so the tested ewe can see her lamb(s) again. Once the ewe has returned near to her lamb(s), a non-familiar shepherd slowly enters the arena through a door located near the grid separating the arena from the litter and stands 20 cm in front of the grid. The third phase of the test (AT3) starts once the shepherd is in place and lasts for 1 min.
Behavioural traits
Scoring test at lambing site: A scoring system, close to those defined by O’Connor et al. (1985), and further validated for hill sheep by Lambe et al. (2001) for use in animal breeding programmes to enable many animals to be scored relatively quickly and easily in extensive sheep systems. The simple scoring system measures maternal reactivity described for each of the two phases described above. In LS1, a maternal behaviour score (LS1-MBS) is recorded on a 5-point scale as follows: 1 - ewe flees and does not return to the lambs within 60 s; 2 - ewe retreats (i.e., at least 2-3 m) but comes back to her lambs within 60 s; 3 - ewe retreats with at least one lamb and comes back; 4 - ewe retreats and returns repeatedly; 5 - ewe stays close to the lambing spot. In LS2, a second maternal behaviour score (LS2-MBS) is recorded on a 4-point scale as follows: 1 - ewe flees; 2 - ewe stays close to the lambing spot, 3 - ewe follows but from a distance (i.e., 1 to 2 m), 4- ewe follows, staying close to the shepherd (i.e., less than 1 m).
Arena test: Locomotor activity and localisation are analysed from the video footage or infrared cells (as described above). Locomotor activity is assessed by measuring the number of zones crossed during the 3 phases (AT1/2/3-LOCOM). The time spent in each zone is recorded. The ewe’s proximity to the litter and/or the human during phases 1 and 3 (AT1/3-PROX) is calculated using the following formula:
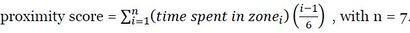
Two types of vocalisations are recorded manually during the test with an electronic device: number of high-pitched bleats are recorded when the animal bleats with an open mouth (AT1/2/3-HBLEAT) and number of low-pitched bleats are recorded when the animal bleats with a closed mouth (AT1/2/3-LBLEAT).
Calculation of traits
Logarithmic transformation is applied to AT1/2/3-LBLEAT to minimise major deviations from the normal distribution. All other elementary variables described above are directly used for genetic analyses.
Use for genetic analysis / genetic evaluation
The (co)variance components for quantitative behavioural traits can be estimated by restricted maximum likelihood (REML) methodology applied in an animal model. The (co)variance components for categorical behaviours can be estimated by MCMC and Gibbs sampling methods using a threshold model (Gilmour et al., 2009).
Assuming that all ewes are measured every year, the analyses assume a repeatability model with behaviour measured across productive cycles considered to be the same trait with a constant variance. Random effects typically include a direct additive and permanent environmental genetic effects of the animal (i.e., ewe).
All relevant fixed effects and interactions should be included in the model. Factors that could be considered can include:
- The litter size at lambing.
- Dam parity or age or age of the dam nested within parity (if significant).
- Contemporary group (e.g., depending on the data collection: flock-year-season effect…).
Behaviour at grazing
Definition, terminology, rationale
Grazing behaviour is a complex combination of various movements and activities of animals in different spatial-temporal scales (Andriamandroso et al, 2016). Indicative traits related to grazing behaviour include:
- Duration of grazing
- Distance walked
- Speed
- Altitude difference
- Elevation gain/loss
- Energy expenditure at grazing
A better understanding of the phenotypic and genetic background of grazing behaviour traits could help towards the development of appropriate breeding programmes to increase adaptation to extensive rearing conditions. However, recording of such traits is challenging. The use of new technologies such as global positioning systems (GPS) could help towards efficiently monitoring grazing behaviour (Homburger et al., 2014; Feldt and Schlecht, 2016).
Data recording
The following guidelines for recording grazing behaviour traits of sheep are based on a study implemented in Greece (Vouraki et al., 2025). Specifically, in the latter study, grazing behaviour of Boutsko sheep reared semi-extensively in mountainous regions was monitored using GPS technology. Moreover, phenotypic and genetic parameters for key grazing behaviour traits were estimated. These guidelines could be enriched in the future based on other relevant studies.
Monitoring of sheep grazing behaviour is performed using appropriate GPS devices attached on designated collars (Figure 3). Rotational monitoring of animals can be applied to reduce the number of devices needed. Selected GPS devices should be of low weight in order to be accepted by the animals without any obvious irritation. Batteries with extended life should be used to provide sufficient energy for GPS tracking for as many as possible consecutive days. In the aforementioned study, “Tractive GPS” devices (Tractive, Pasching, Austria) were used that weighed 28 grams. GPS tracking of each animal was performed for 4-10 days at 2-60 minutes intervals; number of tracking days and intervals were based on available signal and animal movement.
GPS generated data of each animal for the total tracking period are exported in .gpx format. In the case of “Tractive GPS”, the location history function of MyTractive web app (https://my.tractive.com/#/) is used to export recorded data. Then, the exported files are split by date using a designated software such as GPSBabel (version 1.8.0). For each animal, daily routes and corresponding GPS data can be visualized and extracted using appropriate software such as Viking GPS data editor and analyser (version 2.0).
Recorded grazing behaviour traits via these devices include duration of daily grazing (min), distance (km), speed (km/hour), minimum and maximum altitude, and total elevation gain. Other useful metrics including number and average distance between tracking points, tracking duration and route followed by the animals should also be extracted to be used in ensuing analyses.

Calculation of traits
Based on minimum and maximum altitude, altitude difference is calculated. Moreover, energy expenditure for walking can be estimated using the following formula of AFRC (Alderman and Cottrill, 1993):
EE= (0.0026×HD+0.028×VD)×BW
where:
EE = energy expenditure for walking (MJ);
HD = horizontal distance (km, calculated as the difference between distance and elevation gain); VD = vertical distance (km, corresponding to elevation gain);
BW = body weight (kg).
Quality control of GPS generated phenotypes is necessary to sense-check the data for extreme values and errors. Specifically, limits are set for minimum and maximum altitudes to reflect the real altitude of the region being studied. Tracking points beyond these limits are then removed from the corresponding .gpx files and data are re-calculated. Moreover, daily records for which GPS tracking of animals had stopped before returning to their shed, must be excluded. Finally, if needed, grazing behaviour traits should be logarithmically transformed to ensure normality of distribution prior to analyses.
Use for genetic analysis / genetic evaluation
(Co)variance components of grazing behaviour phenotypes and relevant breeding values (EBVs) can be estimated by restricted maximum likelihood methodology applied to an animal mixed model that can include the following random and fixed effects:
Random effects: additive genetic effect and permanent environmental effect of the animal
The relevant fixed effects may include:
- Farm
- Number of GPS tracking points
- Tracking duration
- Distance between tracking points
- Climatic parameters (e.g. temperature-humidity index)
- Sampling time
It may also be desirable to include social grouping (if known), as this can also affect individual animal behaviours.
Acknowledgements
We gratefully acknowledge the contributions to these recording of behaviour guidelines by the following people:
- Dominique Hazard, INRAE, France
- Angeliki Argyriadou, University of Thessaloniki, Greece
- Georgios Arsenos, University of Thessaloniki, Greece
- Alain Boissy, INRAE, France
- Vasileia Fotiadou, University of Thessaloniki, Greece
- Sotiria Vouraki, University of Thessaloniki, Greece
- Joanne Conington, SRUC, the UK
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Alderman, G., Cottrill, B. Energy and Protein Requirements of Ruminants. In An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients; CAB International: Wallingford, UK, 1993.
Andriamandroso, A., J. Bindelle, B. Mercatoris, F. Lebeau (2016). A review on the use of sensors to monitor cattle jaw movements and behavior when grazing. Biotechnologie, Agronomie, Société et Environnement, 20.
Beausoleil, N. J., D. Blache, K. J. Stafford, D. J. Mellor, and A. D. L. Noble. (2012). Selection for temperament in sheep: Domain-general and context-specific traits. Appl. Anim. Behav. Sci. 139:74–85.
Boissy, A., Bouix, J., Orgeur, P., Poindron, P., Bibe, B., & Le Neindre, P. (2005). Genetic analysis of emotional reactivity in sheep: effects of the genotypes of the lambs and of their dams. Genetics Selection Evolution, 37, 381-401. doi:10.1051/gse:2005007
Brand, T. S. (2000). Grazing behaviour and diet selection by Dorper sheep. Small Ruminant Research, 36(2), 147-158.
Brien, F. D., Cloete, S. W. P., Fogarty, N. M., Greeff, J. C., Hebart, M. L., Hiendleder, S., . . . Miller, D. R. (2014). A review of the genetic and epigenetic factors affecting lamb survival. Animal Production Science, 54, 667-693. doi:10.1071/an13140
Cloete, S. W. P., Burger, M., Scholtz, A. J., Cloete, J. J. E., Kruger, A. C. M., & Dzama, K. (2020). Arena behaviour of Merino weaners is heritable and affected by divergent selection for number of lambs weaned per ewe mated. Applied Animal Behaviour Science, 233. doi:10.1016/j.applanim.2020.105152
Dwyer, C. M., Lawrence, A. B. (2005). A review of the behavioural and physiological adaptations of hill and lowland breeds of sheep that favour lamb survival. Applied animal behaviour science, 92(3), 235-260.
Dwyer, C. M. (2008). Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management. Journal of Animal Science, 86, E246-E258. doi:10.2527/jas.2007-0404
Dwyer, C. M. (2014). Maternal behaviour and lamb survival: from neuroendocrinology to practical application. animal, 8, 102-112. doi:doi:10.1017/S1751731113001614
Feldt, T., Schlecht, E. (2016). Analysis of GPS trajectories to assess spatio-temporal differences in grazing patterns and land use preferences of domestic livestock in southwestern Madagascar. Pastoralism, 6(1), 1-17.
Gilmour, A. R., Gogel, B. J., Cullis, B. R., & Thompson, R. (2009). ASReml User Guide Release 3.0 VSN International Ltd, Hemel Hempstead, HP1 1ES, UK. www.vsni.co.uk.
Hazard, D., Moreno, C., Foulquié, D., Delval, E., François, D., Bouix, J., Boissy, A. (2014). Identification of QTLs for behavioral reactivity to social separation and humans in sheep using the OvineSNP50 BeadChip. BMC Genomics, 15, 778. doi:10.1186/1471-2164-15-778
Hazard, D., Bouix, J., Chassier, M., Delval, E., Foulquie, D., Fassier, T., Boissy, A. (2016). Genotype by environment interactions for behavioral reactivity in sheep. Journal of Animal Science, 94, 1459-1471. doi:10.2527/jas2015-0277
Hazard, D., Macé, T., Kempeneers, A., Delval, E., Foulquié, D., Bouix, J., & Boissy, A. (2020). Genetic parameters estimates for ewes’ behavioural reactivity towards their litter after lambing. Journal of Animal Breeding and Genetics, n/a. doi:10.1111/jbg.12474
Hazard, D., Kempeneers, A., Delval, E., Bouix, J., Foulquie, D., & Boissy, A. (2021). Maternal reactivity of ewes at lambing is genetically linked to their behavioural reactivity in an arena test. Journal of Animal Breeding and Genetics, 139, 193-203. doi:10.1111/jbg.12656
Hazard, D., E. Delval, S. Douls, C. Durand, G. Bonnafe, D. Foulquié, D. Marcon, C. Allain, S. Parisot, A. Boissy (2022). Divergent genetic selections for social attractiveness or tolerance toward humans in sheep. WCGALP 2022
Homburger, H., Schneider, M. K., Hilfiker, S., Lüscher, A. (2014). Inferring behavioral states of grazing livestock from high-frequency position data alone. PLoS One, 9(12), e114522.
Keller, M., Meurisse, M., Poindron, P., Nowak, R., Ferreira, G., Shayit, M., & Levy, F. (2003). Maternal experience influences the establishment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Developmental Psychobiology, 43, 167-176. doi:10.1002/dev.10130
Lambe, N R; Conington, J; Bishop, S C; Waterhouse, A; Simm, G (2001). A Genetic Analysis of maternal behaviour score in Scottish Blackface sheep. Animal Science 72: p415-425. Doi:10.1017/s1357729800055922.
Ligout, S., Foulquie, D., Sebe, F., Bouix, J., & Boissy, A. (2011). Assessment of sociability in farm animals: the use of arena test in lambs. Applied Animal Behaviour Science, 135, 57-62. doi:10.1016/j.applanim.2011.09.004
Mignon-Grasteau, S., Boissy, A., Bouix, J., Faure, J.-M., Fisher, A. D., Hinch, G. N., . . . Beaumont, C. (2005). Genetics of adaptation and domestication in livestock. Livestock Production Science, 93, 3-14. doi:10.1016/j.livprodsci.2004.11.001
O’Connor, C. E., Jay, N. P., Nicol, A. M., & Beatson, P. R. (1985). Ewe maternal behaviour score and lamb survival. Proceedings of the New Zealand Society of Animal Production, 45 159–162.
O’Connor, C.E., Lawrence, A. B. and Wood-Gush, D. G. M. (1992). Influence of litter size and parity on maternal behaviour at parturition in Scottish Blackface sheep. Applied Animal Behaviour Science 33: 345–355. https://doi.org/10.1016/S0168-1591(05)80071-1
Simm, G., Conington, J., Bishop, S. C., Dwyer, C. M., Pattinson, S. (1996). Genetic selection for extensive conditions. Applied Animal Behaviour Science, 49(1), 47-59.
SMARTER deliverable D2.4. New prototype and report for industry on GPS-generated phenotypes for behavioural adaptation to extensive grazing systems; artificial rearing adaptation phenotypes; lamb vigour scores linked to lamb survival; new foetal and neonatal survival phenotypes (in preparation).
von Borstel, U. K., Moors, E., Schichowski, C., & Gauly, M. (2011). Breed differences in maternal behaviour in relation to lamb (Ovis orientalis aries) productivity. Livestock Science, 137, 42-48. doi:10.1016/j.livsci.2010.09.028
Vouraki, S., Papanikolopoulou, V., Argyriadou, A., Priskas S., Banos, G., Arsenos, G. (2025). Phenotypic and genetic parameters of grazing behaviour of semi-extensively reared Boutsko sheep. Applied Animal Behaviour Science, vol. 282, Jan 2025, 106473. https://doi.org/10.1016/j.applanim.2024.106473
Guidelines on recording the environment in sheep and goats
Change Summary
| Date of change | Nature of Change |
| October 2024 | First version |
Introduction and scope
Introduction
In the genetic evaluation process, the genetic model includes environmental effects (generally fixed effects, in some cases random effects) to correct the phenotypes from these effects, not related to the genetic value of the animal. These environmental effects that affects the expression of the genotypes depend on the traits and the method of phenotyping, the environment itself (flock/herd, year, parity, season of lambing, number of born or reared lambs/kids, scorer, gender of the lamb/kid, management of mob groups, etc). The quality of the record of the environment is important to correct relevantly the performance of the animal.
Some other environmental effects that are usually included in a general flock/year or management mob group effect could be identified, such as the feeding effect or the climate effect. By including these effects in the genetic model, we could get less biased and more precise EBVs, especially when these effects are individualised or are period-specific (feeding might depend on such and such groups of animals, climate might influence the performance of such and such test- day). Moreover, the more precise knowledge of environmental effect might be valorised for flock/herd management and extension services towards farmers.
Moreover, feeding can be considered as an environmental effect, but as well be constitutive of a performance. This is typically the case for feed efficiency where the quantity and the quality of the diets allows to calculate the phenotype.
Likewise, with the climatic change, breeding for animals more resistant or more resilient to higher temperatures (especially thermal stress) becomes a selection objective per se (example of heat tolerance). In this context, the conditions of temperatures (or temperature/humidity combination) not only might be an environmental factor, but be part of the phenotype.
Other environmental effects can be described and should enrich this document in the future.
Scope
This document focuses on those data that are worth recording the precise the environment or to calculate novel traits of interest.
Following SMARTER work, the document will describe the record of the diet (section 3) and the record of meteorological data (section 4)
Further factors might be described later, letting this document open to new section in the future, including:
- Recording the diet in small ruminant
- Recording meteorological data
- Other environmental records
Recording the diet
Definition, terminology, rationale
Recording the diet consists in collecting data on the quantity and quality of a ration that an animal, a group of animals of a flock/herd consumes at a given period.
The characterisation of the ration, in terms of energy and protein depends upon the countries. For example, the French INRAE Feeding System for Ruminants (Nozière et al., 2018) is different from the British one (AFRC, 1993). This is the reason for which we will describe in this section general recommendations, that can be applied, translated to the domestic feeding system used
Breeding for more efficient animals is more and more important for economic reason (the feeding resources are costly, might be rare in years with climatic excess such as heat or drought) and for environmental reasons (feed/food competition, emission of green-house gases). Feed efficiency is a trait of high interest in this context. Even though it is deceptive to calculate gold standard efficiency trait in private farm, the knowledge of diets in those farms should help to correctly manage the proxies that are promoted in SMARTER. Diet could also be used as a corrective factor in evaluation models in the future. In addition, it might be a support to better understand the herd/flock effect and its variation across year, and therefore give more acute and relevant advice to the farmers.
It is difficult and time-consuming to collect the data for establishing the diet in the flock/herds. The diet is collective in most of the situations (the same amount of forage is given to all animal because the forage is not given individually). When the concentrate is given through Automated Concentrate Feeder (ACF) in the milking parlour, the individualisation is not at the animal scale but at a limited number of groups scale. That’s why we suggest recommendations that must be adapted to each situation.
The aim is to tend to the better possible estimation of the forage ingestion, given that the direct measurement is impossible in commercial farms. Proxies are studied to get indirect measurement of the intake, but they are not validated so far (Near Infra Red Spectra technique). As soon as validated results are available, these recommendations will be updated.
Data recording
When to record the diet
The diet may be recorded at relevant period of the physiological status of the animals in the flock/herd. It is possible to take advantage of the visit of a technician to record the ration (for example when performance recording such as at each (or some of the) test-day when milk recording, or at weighing visit in meat sheep performance recording.
Below are examples of relevant physiological status:
- At mating (or before the mating and after the mating)
- End of gestation (in the month preceding the lambing/kidding)
- After lambing/kidding
- At weaning or just after weaning (peak of production in dairy animals)
- Dairy animals: at each test-day or at some of the test-day
In case of ACF (Automatic Concentrate Feeder), it is possible to record the distribution of concentrate more frequently.
It may be useful to establish the requirements of animals (on average) at each point of diet record. The requirements must concern the energy (in the unit usually used in the country) and the protein (in the unit usually used in the country).
How to record the diet
Individual diet
- This can be obtained through ACF for concentrate, mainly in the milking parlour.
- Intake of forage cannot be collected individually but can be predicted through the intake capacity system, such as the one proposed by INRAE (Nozière et al., 2018).
Collective diet (at the flock/herd scale or at the mob scale)
- Forage (hay, or haylage): some bales of each preservation technic can be weighed once a year with a dry matter (DM) measurement for haylage (it can substantially vary). For hay, DM can be estimated at 85%. Afterward, we can just record how many bales of a given quality (several cutting stages are preserved and not given at random) are distributed per flock per time unit. For silages, it is more complicated, but based on the same procedure, we can weigh one distribution (assuming that it will be constant over time) and simultaneously measured DM. In both situations, if refusals cannot be measured, they must be sufficient for assuming an ad libitum distribution. When the feeding system used in the country can predict the DM intake through the intake capacity of the animal and the quality of the feed, individual diet can be estimated.
- Grazing: for dairy sheep grazing within a short duration per day or the full day, intake can be estimated through ad hoc system. As an example, the new French INRATion feeding software (INRATion V5®) proposes such estimation based on grazing duration, biomass availability and quality.
Defining the constitution of the diet
Precise the type of distribution of the ration
- collective ration
- individual ration (concentrate when ACF)
- pasture
Categories of feedstuff
- Hay
- Partially or fully fermented fodder and fodder preserved by silaging or wrapping:
- Silage
- Wrapped bales
- Pasture
- Straw
- Green feeding
- Dehydrated alfalfa
- Pulp (dehydrated beet pulp, citrus pulp, etc)
- Cake (soybean, rapeseed or sunflower seed)
- Cereals grain (wheat, barley, maize, etc)
- Complete commercial concentrate
- Other by-products of agro-food industry (cereal brans, brewer’s grains, hulls etc.)
Species
For each category, specify the species (rye grass, alfalfa, clover, maize, wheat, barley, etc), physiological stage or age of regrowth, and harvest conditions (cutting length of the forage and added preservative or not for silages, conditions of hay making drying in the field or mechanically dried).
Characterizing the diet
Quantity
Quantity distributed, refused, consumed. Check that these amounts are regularly distributed, refused and consumed because it can markedly influence the animal performance specifically for dairy animals at test day.
The quantity of each feedstuff may be expressed in kg dry matter for forage, in kg gross matter for concentrate. However, final diet for requirement calculation must be expressed as DM.
Requirements
Requirements for the main categories of animals: it depends on the physiological status (maintenance, production, growing, pregnancy)
Average requirement coverage ratio (energy and nitrogen). For example, the requirement coverage ratio in French dairy sheep is roughly 115% for energy and about 125% for nitrogen of the requirements of the average ewe. That allows covering the requirements of about 85-90% of the flock. Difference between energy and nitrogen is assumed to be covered through the body reserve mobilisation.
Quality characterization
The feedstuffs and the ration must be characterized at least in terms of
- Energy
- Protein (or nitrogen)
In case of commercial concentrate, data written on the label are used.
Energy and protein can be expressed in the current unit used in the country. For example, in France, energy is expressed in UFL which is equal to 1.7 Mcal Net energy (Nozière et al., 2018).
It may also be expressed in the international unit, which can be Mcal or MJ.
Use for genetic analysis / genetic evaluation
Diet as part of a phenotype
Calculation of feed efficiency phenotypes: see recommendations on feed efficiency.
Diet as part of a factor in the evaluation model
In most of situations it is impossible in small ruminants to establish individual consumption, for practical reason. The collective effect of the diet is explained in the flock/year effect. The intermediate situation should be when ACF allows to identify several groups within the flock/herd, at a specific test-day or visit. It is possible in this case to put in the model a mob effect grouping animals being given the same amount of concentrate. This should result in a more precise calculation of the breeding value of the animal. Nevertheless, this approach has so far not be used to our knowledge.
Meteorological data
Definition, terminology, rationale
Meteorological conditions may affect the environment effect on the traits of interest. Even though they may be absorbed in a flock effect at the scale of the year or at the scale of a given test-day, it is relevant to be able to quantify the effect of such and such meteorological parameter (and especially the heat stress) ot the zootechnical traits. The global warming and the higher temperature in which the animals are bred emphasises this interest. It is possible to better assess the comfort zone of the populations, that means the meteorological conditions in which the zootechnical traits are not affected. It is also possible to identify animals better adapted to an increase in temperatures or able to be resilient to a wide range of temperatures, that means to maintain their productive ability. In this case, meteorological data, combined with a production trait (growth, milk production, milk composition) or fertility trait, are used as a resilience characterisation by assessing the ability of the animals to recover their production following meteorological challenges.
Meteorological data are mostly temperature, humidity, precipitations, wind speed and radiations. An issue in small ruminants is to select for adapted animals to new environmental challenges, without artificializing their environment of breeding. Mainly because the economic and societal constraints are such as breeding animals outdoors on pasture is desired and breeding indoors inartificialized environment may be costly in terms of energy.
Data recording
Meteorological data from weather station
The aim is to affect outdoors meteorological data to a farm. This can be obtained by assigning to the farm the meteorological data of the closest or more relevant weather stations, using the geographical coordinates of both the farm and the weather station.
The following data may be used:
- Temperature (minimum, maximum, average)
- Relative humidity (amount of moisture in air compared to the maximum amount of moisture it can have at a specific temperature). Expressed in %.
- Specific humidity (ratio of water vapor mass to the total mass of air and water vapor.
- Wind speed
- Precipitations and precipitation type
- Solar radiation
- Atmospheric radiation
- Evapotranspiration
Different index accounting for weather factors have been proposed. One of the most popular is the Temperature Humidity Index (THI) which may be calculated to get a single value representing the combined effects of air temperature and humidity associated with the level of thermal stress.
Different formulas of THI are proposed in the literature. Below is an example of formula proposed by Finocchiaro (et al., 2005):
THI = T − [0.55 × (1 − RH)/100] × (T − 14.4)
where T is the mean daily in °C and RH is the mean relative humidity expressed in percent. Quite often, the parameter used in the analysis model is the temperature of the THI (mainly because temperature and relative humidity are the most available parameters).
Let us also mention the Heat Load Index, referred to as the 'HLI', which is an index that brings together all the weather factors into one number to allow easy interpretation of the cooling capacity of the environment.
The assignation of meteorological data to a farm depends on the countries and on the availability of weather data.
In some countries, the territory may be cut out in a grid, each cell of the grid being considered to have the same meteorological parameters because they are close to the same weather station of reference. As an example, this is the case in France with a grid named SAFRAN cutting the territory into 9892 cells of 64 square kilometres each [8 km by 8 km] (Annex 1). This grid was used, thanks to specific permission from Meteo France, to affect each farm of a given project (by using its GPS coordinate) to a single cell of the grid and thus get relevant meteorological parameters.
The meteorological spatialised data are collected from weather station, on which specific interpolation are applied to present these data on the SAFRAN grid.
The meteorological data key period to consider must be thought according to the production system associated to the breed, type of traits measured and analysed. For example, for milk production (milk recording), we may consider the 3 days preceding the test-day. For semen production, we may consider the meteorological data either at the day of the semen collection, or during the spermatogenesis, which is around 50 days before the semen collection. For the insemination itself (which is in case of fresh semen the same day as semen production), we may consider climate data either the very day of the insemination operation or during a week preceding it.
Environmental data from sensor in the farm
Temperature and humidity may also be collected on site, thanks to sensors situated on-farm, for example in the sheep pen or the stable.
The number of sensors may depend upon the situation and configuration of each building, the goal being to be representative of the pen. In the practical situations of the SMARTER project, 2 to 3 sensors were set in the pen where animals are indoors at a height of 2 meters above the ground, so that they are protected from the animals. If the pen is already equipped by sensors, it is possible to retrieve the data from the existing sensors. The sensors must cover all the relevant groups of animals (primiparous, multiparous, etc), even if they are in different buildings. Measures might be collected several times a day, for example once an hour, to get a precise evaluation of the daily temperature and hygrometry. To relevantly collect the atmosphere of the building, the sensors must be set in a place free from too much air flow or too much sunshine. It is important to regularly check the batteries to avoid loss of data.
Use for genetic analysis / genetic evaluation
Effect of meteorological parameters (eg. temperature or THI) may be estimated on zootechnical traits, using different types of linear models.
The parameter may be considered as a categorical variable (each degree of the parameter being defined as a different class). Or it may be considered in a linear regression on degrees of the parameter.
Reaction norms model, using Legendre polynomial for example, may be used to assess populational losses of the zootechnical trait due to high or low temperature and/or humidity.
Two types of analysis can be made:
- a populational analysis (populational response to the effect of temperature or THI). It gives the comfort rage of each population and how much the loss is with lower or higher temperature or THI.
- an analysis of the genetic components using a random regression model. It permits to estimates genetic parameters of traits according to the temperature or THI and to calculate EBVs of animals at different temperatures or THI levels. Such EBVs allow to identify less vulnerable animals along a range of climate values, so as to identify and select the most robust animals.
Other environmental record
To be completed (or not) when necessary
Acknowledgements
We gratefully acknowledge the contributions to these environment documentation guidelines by the following people:
- Jean-Michel Astruc, IDELE, France
- Antonello Carta, Agris, Italy
- Philippe Hassoun, INRAE, France,
- Gilles Lagriffoul, IDELE, France
- Carolina Pineda Quiroga, NEIKER, Spain
- Eva Ugarte, NEIKER, Spain
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Finocchiaro R, van Kaam JB, Portolano B, Misztal I. Effect of heat stress on production of Mediterranean dairy sheep. J Dairy Sci. 2005 May;88(5):1855-64. doi: 10.3168/jds.S0022-0302(05)72860-5. PMID:15829679.
Nozière, P., Sauvant, D., Delaby, L. 2018. INRA Feeding System for Ruminants. Wageningen Academic Publishers, 640 p., 2018, 978-90-8686-292-4. ⟨10.3920/978-90-8686-292-4⟩. ⟨hal-02791719⟩
AFRC (Agricultural and Food Research Council). 1993. Energy and protein requirements of ruminants. CAB International, Wallingford.
Annexes

Guidelines on recording health and disease in sheep and goats
Change summary
| Date of change | Nature of Change |
| October 2024 | First version |
| December 2024 | Tracked change revisions by MS |
Introduction and scope
Introduction
Livestock diseases cause significant economic losses due reduced productivity, failing to express the genetic potential of animals, treatment costs, and consequently the culling of animals. Therefore, health and resistance to disease are keys factors for increasing resilience in farm animals in general and in small ruminants in particular. Among the challenges that sheep and goats must face, the infectious challenges are among the most important. They lead to losses of production and difficulties of reproduction. They also generate an increase in the consumption of chemical input. Beyond actual extra cost that may hamper the sustainability of the farms, but also of the breeding programs, there is a risk for the environment and the occurrence of resistance to drugs.
In most cases, an integrated approach is the more beneficial and efficient, mixing the different leverages. Among them, the control of the challenges by the host through its genetic resistance has shown its efficiency for some disease (resistance to scrapie, resistance to mastitis in dairy species) or is promising (resistance to parasites, resistance to footrot).
These guidelines on health and disease phenotypes are dedicated to any kind of health and disease resistance indicators. However, to start, we focus on the traits studied in SMARTER, which are the resistance to parasites and the resistance to footrot and mastitis in meat sheep and dairy sheep and goats.
Scope
This section on recording health and disease in sheep and goats starts following the task achieved in SMARTER and includes the following three sub-sections:
- Resistance to parasites
- Resistance to mastitis
- Resistance to footrot
Resistance to parasites
Definition, terminology, rationale
Resistance may be defined as the host’s ability to limit its parasite load (Råberg et al., 2007). The resistance to parasites described here corresponds to the resistance to gastro-intestinal nematodes (GIN). They are one of the main constraints for grazing sheep. They cause substantial economic losses due to lower production levels, the costs of anthelmintic treatments and the mortality of severely affected sheep. GIN control strategies mainly rely on treatment with anthelmintics. In many regions of the world, studies have reported the development of GIN resistance to most anthelmintic molecules due to their extensive use. Additionally, the possible presence of drug residues in animal products and the negative impact of these molecules on the micro and macro fauna of the soil are of concern. Therefore, sustainable GIN control may be a priority with schemes that do not only rely on anthelmintics but include complementary strategies such as nutritional supplementation with tannins and/or proteins, pasture management, and genetic selection of resistant animals. This latter strategy relies on the existence of genetic variation of host resistance to GIN both between and within breeds.
The faecal egg count (FEC), which is the number of parasite eggs per gram of faeces, is the most commonly used indicator to assess this resistance to GIN. In many countries, the selection for parasite resistance is based on FEC measures in natural infestation conditions under natural grazing conditions. As FEC measurements in sheep and goats are extremely costly and laborious, and because response to artificial challenges is highly correlated to response to natural infestation, it is therefore possible to implement a protocol of experimental infestation, as it is the case in France.
Beside FEC, different phenotypes can be used to measure resistance to GINs such as packed cell volume (PCV), FAffa MAlan CHArt (FAMACHA©) score, DAG score, immunological traits, and blood bepsinogen dosing (Shaw et al., 2012; Bishop, 2012; Bell et al., 2019; Sabatini et al., 2023).
Data recording
Indicators of parasite resistance or resilience
Faecal Egg Count
Faecal Egg Count (FEC) is the main indicator that measures the egg excretion intensity. It measures the number of parasite eggs per gram of faeces. This trait is related to the resistance of the animal (ability to limit the installation, the development and the prolificacy of the nematode inside the digestive tract (especially the abomasum). FEC is determined for each sample using a modified MacMaster technique (Whitlock, 1948 or Raynaud, 1970) with a sensitivity of 100 or 15 eggs per gram, respectively. The measure may be done in natural or in experimental infestation. FEC can be applied to one species (for example Haemonchus contortus (Hc)) or several species (including Hc, Teladorsagia circumcincta, Trichostrongylus colubriformis, etc).
The distribution of the FEC has an asymmetric distribution (some high value, many low or medium value). A transformation must be applied to process a genetic analysis. The most frequent transformations are a root (fourth, third or square root) or a log transformation.
Packed Cell Volume
Packed Cell Volume (PCV) - Blood samples were collected in EDTA coated tubes and PCV values were determined individually by centrifugation in microhematocrit tubes with a relative centrifugal force of 9500 for 10 min.
PCV can be exploited as a single value of more relevantly as a gain/loss of PCV between two points. Variation of PCV is a relevant indicator of the resilience of the sheep / goat.
FAMACHA score
FAMACHA® score – As the anaemia provoked by some hematophagous parasites is at some stage visible on the mucosa (especially ocular mucosa), a scale of grading, based on the colour of the ocular mucosa, ranging from 1 (dark red mucosa) to 5 (white mucosa) has been built. This score was developed in South Africa to facilitate the clinical identification of anaemic sheep infected with H. contortus (Van Wyk and Bath, 2002).
As drawbacks, the FAMACHA® score does not allow to detect the non-hematophagous parasites and it appears quite belatedly: a FAMACHA® score over 3 concerns animals with a PCV below 20%. The method is not specific, anaemia being possibly caused by other reason than Haemonchus contortus. It is however interesting to detect the anaemia. FAMACHA® score is related to the resilience of the sheep / goat.
DAG score
DAG score is an indicator for assessing dagginess, which measures faecal soiling in sheep. DAG score uses a 5-point or 6-point scoring scale ranging from 0 (no dags) to 5 or 6 (very daggy). Dag score scale shows the degree or extent of faecal contamination of the fleece.
The key is to be consistent when scoring a mob of sheep and for these sheep to have been run under similar conditions. Faecal contamination should not be confused with urine stain in ewe lambs and hoggets.
Immunological traits
Immunological and physiological profiles may be linked to phenotypes of resistance to parasites (strongyles). These new immunological and physiological profiles are blood lymphocytes cytokine production and serum levels of nematode parasite-specific Immunoglobulin A (IgA) that are produced upon whole blood stimulation. In SMARTER experiment in SRUC, blood was stimulated with pokeweed mitogen (a lectin that non-specifically activates lymphocytes irrespectively of their antigen specificity), and Teladorsagia circumcincta (T-ci) larval antigen to activate parasite-specific T lymphocytes.
Adaptive immune response may be determined by quantifying:
- cytokines interferon-gamma (IFN-γ), which relate to T-helper type 1 (Th1),
- interleukin IL-4, which relates to T-helper type 2 (Th2) and
- interleukin IL-10, which relate to regulatory T cell (Treg) responses.
Each immune trait displays a significant genetic variation (heritabilities ranging from 0.14 to 0.77). Heritability of IgA is moderate (0.41). Correlations with FEC are rather weak, from 0 to 0.27 but not significantly different from 0.
Blood Pepsinogen dosing
Blood pepsinogen is an indicator of the integrity of the gastric mucosa. The determination of serum pepsinogen is therefore a proxy in the diagnosis of abomasal strongylosis of ruminants (pepsinogen in blood is caused by an increase in the permeability of the abomasum mucosa due to presence of nematodes). There is a correlation between the concentration of pepsinogen in the blood and the number of worms harboured by the host.
Natural infestation
General considerations
Measurements (FEC or other proxies) are mainly undertaken in natural infestation under natural grazing conditions. In natural condition of infestation, frequency and amounts of yearly samplings have to be assessed according to the climate and epidemiological conditions and breeds. Local knowledge is essential for adjusting protocols to each country, as the level of infestation is strongly influenced by seasonality and the grazing system.
Several countries (e.g. Australia, New Zealand, and Uruguay), have incorporated the genetic evaluation of FEC at various ages into their national evaluation systems. In any case, in order to have data useful for the genetic evaluation, a representative sample of sheep in the flock involved in the selection scheme has to be periodically monitored to decide whether to sample the whole flock, i.e. when the number of infected animals and the level of infestation are considered sufficient to appreciate individual variability, individual FEC can be measured on the whole flock.
Further data related to environmental factors affecting the level of infestation should be recorded to be included in the genetic model for estimating the breeding values:
- Farm management mainly grazing system
- Birth type
- Sex
- Age of dam
- Parity
- Lambing date
- Sampling date
- Frequency, date, and molecule of anthelmintic administration
Additionally, stool cultures can be performed from the faecal samples taken (one per management group).
Description of the protocol and the measures (Uruguayan protocol)
At weaning, lambs undergo anthelmintic treatment, and their treatment efficacy is checked 8-14 days later through the analysis of FEC samples from 20 randomly selected lambs to confirm the absence of egg excretion. Subsequently, FEC is monitored every 15 days by collecting samples (based on epidemiological conditions) from 10-15% of lambs in each management group. The first individual FEC sampling is conducted when the FEC arithmetic mean exceeds 500 with no more than 20% samples exhibiting zero FEC. At this point, the lambs undergo anthelmintic treatment again, and their treatment efficacy is evaluated after 8-14 days. If the FEC mean remains above 500, a second individual sampling is conducted. Throughout the protocol, faecal egg counts (FEC1 and FEC2) are measured at the end of the first and second natural infestations. Generally, with some variations based on the breed, these samplings correspond to lambs at 9 and 11 months of age, respectively.
Currently, to simplify the protocol, only one sampling is conducted, and the control begins on a fixed date (early autumn) when the most significant parasite, H. contortus, prevails. Along with the FEC records (FEC1 and FEC2), other records, such as body weight, FAMACHA®, and body condition score, can also be taken.
Experimental infestation (French protocol)
As mentioned above, FEC measurements on sheep in commercial flocks are extremely costly and laborious. It has been shown that sheep that are selected on the basis of their response to artificial challenges respond similarly when exposed to natural infestation, and a high positive genetic correlation was estimated between FEC recorded under artificial or natural infestation. Moreover, it has been shown that selection of rams for parasite resistance after artificial challenges allows to improve the resistance of their female offspring for parasite infestation in natural conditions. Thus, an alternative approach may be to select rams gathered for AI progeny-testing or performance-testing by artificially infecting them with standardized doses of larvae.
In most cases, resistance to GIN is assessed in natural infestation conditions at grazing. However, the intensity of natural infestation in grazing animals depends on climatic conditions and may vary from season to season leading to over- or under-estimation of the genetic parameters of resistance. In France, sheep breeds are selected collectively on breeding stations and the strategy is to take advantage of this organization to implement the GIN control selection by phenotyping rams after experimental infestation. There are two main advantages. Firstly, a large diffusion of the genetic progress is expected via these rams, which are the future elite males. Secondly, the experimental infestation performed in control stations allow to evaluate these rams in homogeneous conditions (standardization of doses of infestation, farming conditions, climatic conditions, etc) during the ram evaluation period. Previous studies (Gruner et al., 2004) estimated high genetic correlations between resistances to experimental and natural infestation, between infestation by different parasite species (Haemonchus contortus and Trichostrongylus colubriformis) and between resistance in adult sheep and lambs. Moreover, recent works have shown that the genetic correlation between the resistance of rams in experimental conditions and the resistance of pregnant or milking ewes in natural conditions of GIN infestation are high.
Description of the protocol and the measures
An original protocol for phenotyping resistance to gastro-intestinal parasitism has been conceived and developed in France, targeted to rams (or bucks) gathered in a breeding centre or station, or an AI centre (Jacquiet et al., 2015; Aguerre et al., 2018). Males bred indoors, supposed to be naïve, are artificially infected twice with L3 larvae of a given strain of Haemonchus contortus susceptible to anthelminthic. Males are subjected to a first infestation (after a coprological examination be performed to confirm that no eggs were excreted before the artificial infestation) with a given dose of L3 larvae (D0). At D30, the males are phenotyped (FEC30 and possibly PCV30) and treated with an anthelminthic. After a 15-day recovery period, the rams are challenged again with a given dose of L3 larvae of Haemonchus contortus. At that time (D45), the efficacy of anthelmintic treatment is ensured in each male. Thirty days after (D75) the second challenge, the males are phenotyped (FEC30 and possibly PCV30) and treated again. The protocol lasts 2 and a half months. During the protocol, the measures carried out are as follows:
- faecal egg counts (FEC30 and FEC75) at the end of the first and second infestation (from faecal sample).
- packed cell volumes PCV0, PCV30, PCV45 and PCV75 at the start and the end of both infestation (from blood sample).
Calculation of variables
The FEC30 and FEC75 are used per se. Variations of PCV are calculated:
- PCV_loss_inf1 = PCV0-PCV30 (or ratio PCV30/PCV0)
- PCV_loss_inf2 = PCV45-PCV75 (or ratio PCV75/PCV45)
- PCV_recovery = PCV45-PCV0
where PCV_loss_inf1 and PCV_loss_inf2 represent the loss of PCV after each infestation, while PCV_recovery represents the males’ capacity to recover after the first infestation.
PCV variations might be interpreted as an indicator of resilience of the animal, i.e. its ability to maintain its blood parameters despite the parasitical challenge.
Use for genetic analysis / genetic evaluation
Model for genetic analysis
The genetic analysis of experimentally infected animals that are raised indoors may include:
- Fixed effects: contemporary group (mob x doses of larvae), age of animals (eg. 1 year, 2 years, 3years, 4 years and older)
- Random additive effect of the animals
- Residual effect
The genetic analysis of naturally infected animals that are raised outdoors may include:
- Fixed effects: they obviously will depend of the type of animals (females in lactation vs lambs/kids). They should include flock/herd, year x season (e.g. spring, summer, autumn, winter), anthelmintic treatments (e.g. eprinomectin, ivermectin, moxidectin …) in interaction with the number of days between the date of treatment and the sampling date (e.g. less than 70 days, between 70 and 100 days, more than 100 days). For females in lactation: age and/or parity, litter size before lactation (single or multiple new-born lambs). For lambs or kids: age of the dam, type of birth or rearing, and age at the time of the records, expressed in day.
- Random additive effect of the animals
- Random permanent environment effect if repeated measures (e.g. for FEC 1 & 2)
- Residual effect
Genetic parameters
Pooled estimates of heritability to resistance to gastrointestinal parasites gained by meta-analysis (Mucha et al., 2022) in dairy goats and sheep and meat sheep are shown in Tables 1 and 2, while Table 3 shows the heritabilities estimated for the experimentally infected rams. In addition, we mention a paper from Casu et al (2022) in which a heritability of 0.21 for FEC was found in a 20 year follow-up study in an experimental flock in Sardinia, Italy.
Table 1. Pooled estimates of heritability of resistance to gastrointestinal parasites from meta- analysis in dairy goats and sheep.
| Trait1 | Species | Pooled h2(±SE) | Min2 h2 | Max3 h2 | N obs | N studies |
| FEC | Goats | 0.07 ± 0.01 | 0.04 | 0.15 | 8 | 2 |
| Sheep | 0.14 ± 0.04 | 0.09 | 0.35 | 6 | 3 |
1Trait: FEC – faecal egg count
2Minimum h2 from individual studies included in meta-analysis
3Maximum h2 from individual studies included in meta-analysis
Table 2. Pooled estimates of heritability of resistance to gastrointestinal parasites from meta- analysis in meat sheep (Mucha et al., 2022).
| Trait1 | Pooled h2 (±SE) | Min2 h2 | Max3 h2 | N obs | N studies |
| DAG | 0.30±0.06 | 0.06 | 0.63 | 37 | 15 |
| FCons | 0.14±0.02 | 0.03 | 0.27 | 13 | 5 |
| NBW4 | 0.10±0.02 | 0.00 | 0.54 | 11 | 3 |
| Par-Ab | 0.18±0.07 | 0.05 | 0.29 | 6 | 3 |
| Par-Ig | 0.36±0.06 | 0.13 | 0.67 | 24 | 8 |
| FEC | 0.29±0.03 | 0.00 | 0.82 | 118 | 32 |
| HC | 0.32±0.14 | 0.08 | 0.56 | 5 | 2 |
1Trait: DAG – dagginess, FCons – faecal consistency, NBW – number of worms, Par-Ab – parasitism anitbodies, Par-Ig – parasitism immunoglobulin, FEC –faecal egg count, HC - Haematocrit
2Minimum h2 from individual studies included in meta-analysis
3Maximum h2 from individual studies included in meta-analysis
4Pooled heritability obtained from a simple random effects model as the three level meta-analysis model did not converge
Table 3. Estimates of heritability of resistance to gastrointestinal parasites from meta-analysis in dairy sheep in experimental infestations (Aguerre et al., 2018)
| Trait1 | h2 |
| Root FEC_inf1 | 0.14±0.04 |
| RootFEC_inf2 | 0.35±0.08 |
| PCV_loss_inf1 | 0.24±0.05 |
| PCV_loss_inf2 | 0.18±0.06 |
| PCV-recovery | 0.16±0.06 |
Resistance to mastitis
Definition, terminology, rationale
In small ruminants, mastitis mainly consists in subclinical infections caused by coagulase- negative staphylococci, which is much more frequent than clinical mastitis (Bergonier et al., 2003). Under these conditions, somatic cell count (SCC) is an accurate, indirect measure to predict mammary gland infection. Therefore, SCC could be used as an indirect selection criterion for mastitis resistance as is widely done in dairy cattle. Moreover, selection for mastitis resistance in dairy sheep and goats could mainly focus on selection against subclinical mastitis using SCC, considering the low incidence of clinical cases in these species (<5%), compared to dairy cattle for which clinical cases occur frequently (Bergonier et al., 2003).
Clinical mastitis is not recorded in dairy small ruminants, mainly because of its low incidence and because SCC is a relevant and simple indicator of intra-mammary infections. Work completed in France has developed two lines of ewes (experimental farm INRAE-La Fage) and goat (experimental farm INRAE-Bourges), a high line generated from sires with unfavourable EBVs for somatic cells and a low line generated from sires with favourable EBVs for somatic cells. For both sheep (Rupp et al., 2009) and goats (Rupp et al., 2019), the low line has the lowest SCC, the lowest incidence of clinical mastitis and the lowest incidence of chronic mastitis (abscesses or unbalanced udder) and subclinical mastitis (assessed by milk bacteriology).
Even though SCC is the established indicator for use in animal breeding, the use of the California Milk test (CMT) is a very good indicator of SCC for monitoring udder health in flock/herd management in dairy and meat-producing small ruminants.
Data recording
Somatic Cell Count (SCC)
Large scale somatic cell counting relies on the application of routine methods, such as fluoro- opto-electronic counting. The somatic cell counter must be properly calibrated against a reference and laboratories must frequently verify the calibration settings are still correct.
The design for recording SCC will depend upon the objective. For flock/herd management related to high bulk SCC, the whole flock/herd should be sampled and analysed to identify the animals with the highest SCC. For genetic purpose, simplified designs might be implemented.
In dairy species, somatic cell counting is achieved within the milk recording design and the sampling design, as for milk components such as fat and protein content. As in small ruminants, most of the designs are simplified ones compared to the A4 method (all daily milkings recorded, once a month) (see ICAR Guidelines Section 16: dairy sheep and goats), SCC are quite often available at one out of the two daily milkings. In this case, use of SCC must be handled accordingly.
As for milk composition, with the aim of simplifying and decreasing further the cost of recording, it is possible/recommended to measure SCC on only a part of the flock/herd (first parity or first two parities). It is also possible to go further in the simplification of the design, for example by sampling only a part of the lactation within a part-lactation sampling as proposed in the section 16 of the ICAR Guidelines. The genetic parameters of test-day and lactation mean for Somatic Cell Score (SCS - log-transformed SCC) show that the records of the middle of the lactation appear as the most representative of the whole lactation. Two to four individual samples per female and per lactation, collected monthly in the middle part of the lactation are highly correlated (around 0.98) with SCS determined from samples collected over the complete lactation (A4 method) but are hardly less heritable compared with the A4 homologous traits (negligible loss of precision for SCS) (Astruc and Barillet, 2004). The balance between cost and genetic efficiency, depending on the genetic correlations close to 1, is clearly in favour of the part-lactation sampling compared to A4 testing.
California Mastitis Test (CMT)
The California mastitis test is an animal-side diagnostic test that provides an estimate of the level of infection within a mammary gland. A sample of milk (~3ml) from each udder half is combined with an equal volume of reagent in a CMT paddle and mixed for 15 to 20 seconds. The reaction is scored based on the level of thickening of the mixture from zero (no thickening) consistent with no, or low, levels of infection, to four (gel formation with elevated surface) indicating high levels of infection.
A previous study (McLaren et al., 2018) has demonstrated the strong correlation between CMT score and SCC from samples collected from pedigree meat sheep in the UK.
Calculation of traits
Test-day SCC must be transformed to Somatic Cell Score (SCS) by the logarithmic transformation of Ali and Shook (1980) to achieve normality of distribution.
Example: SCS = log2+(SCC/100,000)+ 3
The table 4 gives correspondence between SCC and SCS
Table 4. Correspondence between somatic cell score and somatic cell count
| Somatic Cell Count (SCC) | Somatic Cell Score (SCS) |
| 12,500 | 0 |
| 25,000 | 1 |
| 50,000 | 2 |
| 100,000 | 3 |
| 200,000 | 4 |
| 400,000 | 5 |
| 800,000 | 6 |
| 1,600,000 | 7 |
SCS can be adjusted for days-in-milk (DIM). In this case, the adjustment procedure must be defined from a study based on healthy ewes/goats with enough number of test-days over the lactation. Then a lactation SCS (LSCS) may be calculated (case of lactation model in genetic evaluation).
LSCS can be computed as the weighted arithmetic mean of test-day SCS (adjusted or not for DIM). Weights are either 1 (equivalent to no weight) or r2, where r is the correlation between one measure and the mean of all other records.
Use for genetic analysis / genetic evaluation
Genetic model
The genetic model might include the following fixed effects:
- Flock x year (x parity)
- Month of lambing/kidding
- Age at lambing/kidding
- Number of lambs/kids born
Genetic parameters
Pooled estimates of heritability of somatic cell score gained by meta-analysis (Mucha et al., 2022) in dairy goats and sheep and meat sheep are shown in Table 5.
Table 5. Pooled estimates of heritability of somatic cell score from meta-analysis in dairy goats and sheep (Mucha et al., 2022)
| Trait1 | Species | Pooled h2 (± SE) | Min2 h2 | Max3 h2 | N obs | N studies |
| SCS | Goats
Sheep |
0.21±0.01
0.13±0.02 |
0.19
0.03 |
0.24
0.27 |
5
29 |
3
22 |
1Trait: SCS – somatic cell score
2Minimum h2 from individual studies included in meta-analysis
3Maximum h2 from individual studies included in meta-analysis
Table 6. Pooled estimates of genetic correlations (rg) between resilience (SCS, FEC) and efficiency (MY, FC, PC) traits from meta-analysis in dairy goats (Mucha et al., 2022)
| Traits1 | Pooled rg (± SE) | Min2 rg | Max3 rg | N obs | N studies |
| SCS & MY | 0.35±0.31ns | 0.00 | 0.59 | 3 | 3 |
| SCS & FC4 | -0.19±0.01 | -0.20 | -0.18 | 3 | 2 |
| SCS & PC | -0.06±0.05ns | -0.13 | 0.00 | 3 | 2 |
| FEC & MY | 0.17±0.35ns | -0.21 | 0.63 | 4 | 2 |
1Traits: SCS – somatic cell score, FEC – faecal egg count, MY – milk yield, FC – fat content, PC – protein content
2Minimum rg from individual studies included in meta-analysis.
3Mmaximum rg from individual studies included in meta-analysis.
nspooled correlations obtained from a simple random effects model as the three level meta-analysis model did not converge
Table 7. Pooled estimates of genetic correlations between resilience (SCS) and efficiency (MY, FY, PY, FC, PC) traits from meta-analysis in dairy sheep (Mucha et al., 2022)
| Traits1 | Pooled rg (± SE) | Min2 rg | Max3 rg | N obs | N studies |
| SCS & MY | -0.05±0.10ns | -0.30 | 0.23 | 16 | 11 |
| SCS & FC | 0.04±0.05ns | -0.16 | 0.16 | 8 | 8 |
| SCS & PC | 0.12±0.03 | 0.02 | 0.24 | 12 | 9 |
| SCS & FY | 0.11±0.15ns | -0.04 | 0.31 | 4 | 4 |
| SCS & PY | 0.17±0.10ns | 0.06 | 0.31 | 4 | 4 |
1Traits: SCS – somatic cell score, MY – milk yield, FY – fat yield, PY – protein yield, FC – fat content, PC – protein content
2Minimum rg from individual studies included in meta-analysis
3Maximum rg from individual studies included in meta-analysis
nsPooled correlations obtained from a simple random effects model as the three level meta-analysis model did not converge
ns – Pooled estimate did not differ significantly from zero
Table 8. Estimates of heritability of somatic cell score, clinical mastitis and CMT in meat and dairy and meat sheep (source Oget et al., 2019)
| Sheep | Breed | Trait1 | Heritability (±SE) | Reference |
| Dairy | Chios | CMT | 0.12±0.06 | Banos et al., 2017 |
| Meat | Belclare, Charollais, Suffolk, Texel,
Vendeen breeds |
CM | 0.04±0.03 | O’Brien et al., 2017 |
| Meat | Texel | SCS | 0.11±0.04 | McLaren et al., 2018 |
| Meat | Texel | CMT | 0.08-0.09±0.04 | McLaren et al., 2018 |
| Meat | Texel | CMT | 0.07 | Kaseja et al., 2023 submitted paper (SMARTER, D2.3) |
1CMT - California mastitis test, CM - Clinical mastitis (examination and palpation of the udder), SCS – Somatic Cell Score
Resistance to footrot
Definition, terminology, rationale
Footrot is caused by Dichelobacter nodosus and is a major cause of lameness in sheep. The disease is highly contagious and endemic in many countries that causes pain and welfare issues in affected animals. In addition to the direct impacts on time and veterinary / medicine costs, the disease has further, indirect, impacts through reducing fertility and milk supply.
The presence of footrot is assessed by inspection of the hooves of lame animals.
Data recording
Scoring methods
Each hoof is assessed individually and scored based on the five-point scale (used in UK): clean, unaffected hoof (score 0), mild inter-digital inflammation (score 1), inter-digital necrosis (score 2), under-running of the sole of the hoof (score 3) and fully under-run to the abaxial wall of the hoof (score 4) (Conington et al., 2008).
The sum of scores is calculated by adding all four scores (for each hoof), hence the animal can obtain the phenotype in a range from zero to 16.
In France, where footrot is usually not recorded, a simplified scoring system has been developed using a scale (0 normal and severity of lesions scored from 1 to 3) adapted from the Victorian Farmers Federation and Coopers Animal Health.
Additionally, the health of feet is assessed in France and the UK for other important hoof lesions including white line degeneration, contagious ovine digital dermatitis, horn growth, presence of abscess, granuloma, interdigital hyperplasia, and panaritium).
Calculation of traits
Sum of scores are log-transformed in order to normalise the data using the formula ln(Sum of scores + 1). The addition of one prevents to logarithm the value of sum of scores equal to zero. Each animal can obtain transformed score ranging between zero and 2.83.
Use for genetic analysis / genetic evaluation
Genetic model
The genetic model might include the following fixed effects:
- Age of the dam
- Scorer (if more than one)
- Vaccine status (if some animals treated with the vaccination against ovine foot-rot)
- Flock or Flock x Year interaction
Genetic parameters
The estimated heritability for UK meat sheep (Table 9) varies between 0.12 (Nieuwhof et al., 2008). to 0.23 (Kaseja et al., 2023, unpublished results) Table 9. Estimates of heritability of resistance to footrot in meat sheep breeds.
Table 9. Estimates of heritability of resistance to footrot in meat sheep breeds.
| Breed | Trait1 | Heritability (SE) | Reference |
| Texel | RF | 0.12(0.02) | Kaseja et al, 2023 in press |
| Scottish Blackface | CM | 0.19 to 0.23 | Kaseja et al., 2023 in press. |
| Scottish lambs | SCS | 0.12 | Nieuwhof et al., 2008 |
| Texel | CMT | 0.18 | Mucha et al., 2015 |
1RF - Resistance to footrot, CM - Clinical mastitis (examination and palpation of the udder), SCS – Somatic Cell Score, CMT - California mastitis test
Acknowledgements
We gratefully acknowledge the contributions to small ruminant health and disease guideline by the following people:
- Joanne Conington, SRUC, the UK
- Jean-Michel Astruc, IDELE, France
- Rachel Rupp, INRAE, France
- Beat Bapst, Qualitas AG, Switzerland
- Donagh Berry, TEAGASC, Ireland
- Beatriz Carracelas, INIA, Uruguay
- Antonello Carta, Agris Sardegna, Italy
- Gabriel Ciappesoni, INIA, Uruguay
- Arnaud Delpeuch, IDELE, France
- Frédéric Douhart, INRAE, France
- Karolina Kaseja, SRUC, the UK
- Ed Smith, The British Texel Sheep Society, the UK
- Flavie Tortereau, INRAE, France
- Stefen Werne, FiBL, Switzerland
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
This work also used deliverable from the Eurosheep project (Horizon 2020 under agreement N° 863056).
References
Aguerre, S., Jacquiet, P., Brodier, H., Bournazel, J.P., Grisez, C., Prévot, F., Michot, L., Fidelle, F., Astruc, J.M., Moreno, C.R. (2018). Resistance to gastrointestinal nematodes in dairy sheep: Genetic variability and relevance of artificial infection of nucleus rams to select for resistant ewes on farms. Vet. Parasitol. 256:16-23. https://doi.org/10.1016/j.vetpar.2018.04.004
Ali, A., Shook, G. (1980). An optimum transformation for somatic cell concentration in milk. J. Dairy Sci. 63:487-490.
Astruc J.M., Barillet F. (2004). Current challenge for milk recording in dairy sheep and goats: the simplification of milk sampling design for chemical composition and somatic cell counts of milk. Proceedings of the 34th ICAR session, Sousse, Tunisia, 31 May-3 June 2004.
Banos, G., Bramis, G., Bush, S.J., Clark, E.L., McCulloch, M.E.B., Smith, J., Schulze, G., Arsenos, G., Hume, D.A., Psifidi, A. (2017). The genomic architecture of mastitis resistance in dairy sheep. BMC Genom. 18 624.
Bell, A., McNally, J., Smith, D.V., Rahman, A., Hunt, P., Kotze, A.C., Dominik, S., Ingham, A. (2019). Quantification of Differences in Resistance to Gastrointestinal Nematode Infections in Sheep Using a Multivariate Blood Parameter. Vet. Parasitol. 270:31–39.
Bishop, S.C. (2012). Possibilities to Breed for Resistance to Nematode Parasite Infections in Small Ruminants in Tropical Production Systems. Animal., 6:741–747.
Bergonier, D., Crémoux, R. de, Rupp, R., Lagriffoul, G., Berthelot, X. (2003). Mastitis of dairy small ruminants. Vet. Res. 34:689–716.
Casu, S., Usai, M.G., Sechi, T. et al. Association analysis and functional annotation of imputed sequence data within genomic regions influencing resistance to gastro-intestinal parasites detected by an LDLA approach in a nucleus flock of Sarda dairy sheep. Genet Sel Evol 54, 2 (2022). https://doi.org/10.1186/s12711-021-00690-7
Conington, J., Hosie, B., Nieuwhof, G.J., Bishop, S.C., Bünger, L. (2008). Breeding for resistance to footrot- the use of hoof lesion scoring to quantify footrot in sheep. Vet. Res. Commun. 32(8):583-9. https://doi.org/10.1007/s11259-008-9062-x
Gruner, L., Bouix, J., Brunel, J.C. (2004). High genetic correlation between resistance to Haemonchus contortus and to Trichostrongylus colubriformis in INRA 401 sheep. Vet. Parasitol. 119:51–58.
Jacquiet, P., Salle, G., Grisez, C., Prevot, F., Lienard, E., Astruc, J.M, Francois, D., Moreno, C. (2015). Selection of sheep for resistance to gastro-intestinal nematodes in France: where are we and where are we going? 25th International Conference of the WAAVP, Liverpool, UK, 2015, 16-20 August
McLaren, A., Kaseja, K., Yates, J., Mucha, S., Lambe, N.R., Conington, J.(2018). New mastitis phenotypes suitable for genomic selection in meat sheep and their genetic relationships with udder conformation and lamb live weights. Animal. 12(12):2470-2479. doi: 10.1017/S1751731118000393.
Mucha, S., Bunger, L., Conington, J. (2015). Genome-wide association study of footrot in Texel sheep. Genetics Selection Evolution, 47 (1), pp.35. DOI 10.1186/s12711-015-0119-3
Mucha, S., Tortereau, F., Doeschl-Wilson, A., Rupp R., Conington, J. (2022). Animal Board Invited Review: Meta-analysis of genetic parameters for resilience and efficiency traits in goats and sheep. Animal. 16(3):100456. https://doi.org/10.1016/j.animal.2022.100456.
Nieuwhof, G.J., Conington, J., Bünger, L., Haresign, W., Bishop, S.C. (2008). Genetic and phenotypic aspects of resistance to footrot in sheep of different breeds and ages. Animal. 2(9):1289-1296. https://doi.org/10.1017/S1751731108002577
O’Brien, A.C., McHugh, N., Wall, E., Pabiou, T., McDermott, K., Randles, S., Fair, S., Berry, D.P. (2017). Genetic parameters for lameness, mastitis and dagginess in a multi-breed sheep population. Animal 11, 911–919. DOI: 10.1017/S1751731116002445
Oget, C., Tosser-Klopp, G., Rupp, R. (2019). Genetic and genomic studies in ovine mastitis. Small Ruminant Research 176, 55-64. https://doi.org/10.1016/j.smallrumres.2019.05.011.
Råberg, L, Sim, D., Read, A.F. (2007). ‘Disentangling genetic variation for resistance and tolerance to infectious diseases in animals’, Science. 318(5851), 812-814 DOI: 10.1126/science.1148526
Raynaud J.P. (1970). Etude de l’efficacité d’une technique de coproscopie quantitative pour le diagnostic de routine et le controle des infestations parasitaires des bovins, ovins, equines et porcins. Ann. Parasitol. 45: 321–342
Rupp, R., Bergonier, D., Dion, S., Hygonenq, M.C., Aurel, M.R., Robert-Granié, C., Foucras, G. (2009). Response to somatic cell count-based selection for mastitis resistance in a divergent selection experiment in sheep. J. Dairy Sci. 92, 1203–1219.
Rupp, R., Huau, C., Caillat, H., Fassier, T, Bouvier, F., Pampouille, E., Clément, V., Palhière, I., Larroque, H., Tosser-Klopp, G., Jacquiet, P., Rainard, P. (2019). Divergent selection on milk somatic cell count in goats improves udder health and milk quality with no effect on nematode resistance. J Dairy Sci. 102(6):5242-5253. doi: 10.3168/jds.2018-15664.
Sabatini, G.A., de Almeida Borges, F., Claerebout, E. et al. (2023). Practical guide to the diagnostics of ruminant gastrointestinal nematodes, liver fluke and lungworm infection: interpretation and usability of results. Parasit. Vectors. 16, 58. https://doi.org/10.1186/s13071-023-05680-w
Shaw, R.J., Morris, C.A., Wheeler, M., Tate, M., Sutherland, I.A. (2012). Salivary IgA: A Suitable Measure of Immunity to Gastrointestinal Nematodes in Sheep. Vet. Parasitol. 186, 109–117
Van Wyk, J.A., Bath, G.F. (2002). The FAMACHA© system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet. Res. 33:509–529.
Whitlock, H.V. (1948). Some modifications of the McMaster helminth egg counting technique and apparatus. J. Coun. Sci. Ind. Res. 21:177.
Annexes




Guidelines on recording lifetime resilience in sheep and goats
Change Summary
| Date of change | Nature of Change |
| October 2024 | First version |
| November 26th 2024 | Comments made by JC |
| December 23rd 2024 | Comments made by MS |
Introduction and scope
Introduction
Lifetime resilience is often tackled through longevity and aspects of productive longevity. Longevity is a trait to quantify productive lifespan of livestock, and for increasing durability and profitability of farms. In dairy ruminants, longevity definitions include: (i) true longevity (all culling reasons, including milk productivity); and (ii) functional longevity (all culling reasons, except voluntary productivity, such as milk productivity or growth). Functional longevity (corrected for production level – milk, growth) reflects the animals’ accumulated ability to overcome health and nutritional challenges. It is an indirect global approach to quantify adaptive capacity to various production environments. Different indicators may be calculated. One indicator is the length of productive life which is computed as the time interval (in days) between first lambing/kidding and culling. Longevity is linked with various predictors, such as fertility, udder health and conformation, resistance to disease, body condition score changes across ewe/doe lifetime. These predictors may be used in breeding program to get an earlier breeding value of longevity and may help to manage and monitor lifetime resilience at the farmer level.
Scope
The scope of these guidelines is to define approaches for the definition of longevity as well as the traits that can be calculated, and the downstream analyses that can be set up (including the use of early predictors to enhance longevity in the evaluation process).
To propose a grid for setting up an observation of the culling causes.
Longevity
Definition, terminology, rationale
The notion of longevity can cover several meanings. Longevity can be understood as the true longevity, i.e. the ability of the animal to live as long as possible, whatever its production level and its functional characteristics. Animal longevity also depends on the replacement rate which is often a choice of the breeders. Animals may be culled due to production level such as milk production or growth or fat/muscle depth, leading to ’voluntary’ culling (i.e. an animal is culled because we want to do it). In contrast, ‘involuntary culling’ is defined as an animal having to leave the flock or herd due to illness / accident/ functional disability etc (i.e. they are culled because we have to do it)
Involuntary culling may be due to:
- Udder health problem (clinical, subclinical, chronic mastitis).
- Lack of resistance to disease such as parasites.
- Problem of footrot.
- Unfavourable shape of the udder (lack of adaptation to machine milking or to suckling).
- Unfavourable general conformation.
- Undesired behaviour (temperament in the milking parlour).
- Infertility or any problem of reproduction.
- Problem of feet or legs, lameness.
- Lack or excess of body tissue mobilisation.
any other undesirable aspect associated with the animal’s inability to produce. Voluntary culling may be due to:
- Low productivity,
- Management decision to cull for age,
- Management decision to cull for a specific coat colour / other phenotype that does not meet the type desired,
- Farmer doesn’t like the animal,
- Economic reason to reduce the number of breeding animals in the flock/herd.
Even if some of these reasons for culling may be considered per se in the selection process by phenotyping and evaluating related traits (for example resistance to mastitis, resistance to gastro- intestinal parasite, fertility, udder morphology), it is often not possible to account for all of them. If properly modelled, functional longevity may be considered as a global and composite approach, allowing to assess the sustainability of the population in selection and of the practiced selection.
For this, different traits may be considered, quite often they are relatively easy to compute with data usually already existing in the genetic database (ex. length of productive life, which can be calculated as the culling date minus the date of the first lambing). There is no additional recording to set up. The difficulties in handling functional longevity are related to the modelling of the trait, given that the trait is fully known when the animal is culled. When not yet culled, the model to set up are quite complex. An example of this was reported by Brotherstone et al. (1997) for dairy cattle and Conington et al. (2004) for hill sheep, whereby live animals’ EBVs for longevity are based on their probability of survival at a given age combined with actual cull dates of relatives that became breeding females in the flock.
Even though there is no need to identify/know the cause of culling, the knowledge of the cause of culling might be a relevant observation of the hierarchy of the culling cause, which may lead to put an emphasis on some specific issue. For example, if we observe an increase in some culling causes (let’s say parasitism) this should lead to a deliberate selection programme to breed more resistant animals to parasites.
One drawback of the functional longevity trait is its lack of precocity. As stated above, it is necessary to have the date of culling or to have accumulated enough lactation to compute the trait. And an appropriate model (e.g. survival analysis) can only partially disentangle this difficulty. It is possible to address this issue by running a multi trait genetic evaluation model combining the longevity trait and some other proxy traits (such as udder morphology, udder health, etc). The use of Genomic Selection is a complementary way to generate early prediction of genetic merit for longevity, provided there is good accuracy of the EBVs of animals in the associated reference population.
Data recording
Longevity traits
The table 1 presents some criteria commonly used in small ruminants to measure longevity. Here, the criteria deal with true longevity, the only one measurable in herd/flocks. Functional longevity will be estimated later, at the statistical analysis step. Table 1 also shows the data required for calculating the longevity criteria. For example, the length of productive life is referred to as the difference between the time a female enters the breeding flock/herd and the date she exits it due to being culled or dying. It is important to notice that the culling date, which is rarely recorded by the farmers, can be replaced by the date of the last event registered for the animal (for example, date of the last performance recording, or of the last reproduction event).
Table 1. Definition of some commonly used longevity criteria
| Longevity criteria | Raw data required | Calculation |
| Length of total lifespan (LTL) | Birth date (BD)
Culling or death date (CD) |
LTL= CD - BD
in days (or months or years) |
| Length of productive life (LPL) | First lambing/kidding date (FKD)
Culling or death date (CD) |
LPL = CD – FKD
in days (or months or years) |
| Total number of days in production (NDL) | Days in milk per lactation (DIM)
or Lambing/kidding date + dry off date for each lactation |
NDL = ∑ DIM |
| Number of lactations (NLACT) | Each lambing/kidding event (KE) | NLACT = ∑ KE |
| Number of lambs or kids during lifetime (NLAMB) | Prolificacy at each lambing/kidding (PR). This may or may not include no. lambs born dead + no. lambs born alive | NLAMB = ∑ PR |
The length of total lifespan can be estimated easily, with only two variables usually registered by farmers. The difference with the length of productive life is that it considers the period when animals had the first lambing/kidding as well as the lambing/kidding interval. If the age at the first lambing/kidding and the lambing/kidding interval are similar between animals, the length of total lifespan will be very close to the length of productive life.
The total number of days in production only covers the “useful” life of the females because it doesn’t include the unproductive periods (such as dry off or large lambing/kidding interval after reproduction failure), compared to length of productive life. But the number of variables necessary to compute it is larger.
For the total number of lambs or kids during a lifetime, it is necessary to include all live-born lambs/kids only or those reared to weaning, if these data are routinely recorded.
Calculation of traits
The last column of Table 1 indicates how to calculate the different longevity criteria, from the raw variables.
The length of total lifespan and the length of productive life are estimated as differences in days between two dates: i) the culling date and ii) the birth date or the first lambing/kidding date, respectively. The total number of days in production corresponds to the sum of the days in milk of each lactation of the female. For the last two criteria (number of lactations or number of lambs/kids), the estimation corresponds to cumulative performance across lifetime.
Instead of waiting for the end of the animal's life to calculate the longevity criterion (which is sometimes long), one solution deals with limiting the animal career to a maximum number of years or lactations. For example, the length of productive life can be calculated only on the first 6 lactations. Subsequently, the length of productive life will be defined as the total number of days between the first lambing/kidding and the end of the 6th lactation. In the same way, the total number of lambs/kids can be estimated at a fixed age, 8 years old for example.
Use for genetic analysis / genetic evaluation.
Models
The genetic ability for longevity is evaluated via the functional longevity, i.e. the true longevity corrected for production traits. Functional longevity is defined at this step, by integrating the level of production as fixed effect in the analysis of longevity criteria described in Table 1.
Different methods are used for the genetic evaluation of longevity traits.
The first method is based on linear models. The main advantage of these models is their ease of implementation because they are used for most of the traits under selection. But they have different drawbacks regarding longevity:
- they do not fit well longevity because longevity indicators do not follow a normal distribution
- they consider only animals that have finished their productive life (unless separate predictors are used). This has two consequences: the longevity data are skewed if living animals are ignored; the breeding value is available lately in the life of the animals. This is notably the case for males for whom most of their offspring must be culled to be evaluated.
- they are not able to include time-dependant variables (e.g. parity, lactation stage). Time dependant variables are useful to take into account the changes in breeding conditions that occur during the life of the animal, and thus to better model longevity data.
The second method is based on proportional hazard model or survival analysis. This type of model counterbalances all the drawbacks of linear models and thus, are the best ones to estimate breeding values for functional longevity. Nevertheless, they are complicated to implement in a routine genetic evaluation process, and a few software exist for genetic survival analyses such as Survival kit, (Ducrocq et al, 2005). However, an evaluation based on an animal model is not feasible in large dataset, leading to use sire-maternal grand-sire models or sire models. Under this assumption, ewes/does EBVs are not available (Ducrocq, 2001).
A third method, less widespread, considers the first three lactations as separate traits in a multiple trait animal linear model. Each lactation is assigned to 1 (instead of 0) once the female reaches the next lactation.
Factors of variation
The main factors of variation of longevity data are:
- herd/flock
- year
- kidding/lambing season
- birth season
- age at first lambing/kidding
- breed
- herd/flock size and herd/flock size variation
- lactation stage, parity (if survival analysis model)
- number of lambs/kids born and reared (for meat sheep and goats)
- within herd/flock production level: this factor of variation is essential to integrate to estimate the functional longevity. Usually, it is the within herd/flock level of production (and not the absolute level of production) that is considered because it explains the decision of the breeder to cull the animal.
Heritabilities of functional longevity
Heritabilities range between 5% and 17% (Sasaki, 2013, Castañeda-Bustos et al., 2014, Geddes et al., 2017, Palhière et al (2018), Buisson et al (2022), Pineda-Quiroga & Ugarte, 2022) indicating that this trait has a low to moderate genetic background. This might be due to the composite signification of longevity, which represents a synthesis of various abilities (see § on predictors).
However, the genetic variation coefficients are moderate suggesting that a genetic variability may be exploited to set up a selection programme.
Genetic correlations
The genetic correlations between functional longevity and other traits are:
- close to 0 for milk production traits. This results from the model, in which longevity is corrected for level of production,
- from 0 to 0.40 for udder type traits (Castañeda-Bustos et al., 2014). The rear udder attachment and the udder floor position are the most correlated to functional longevity,
- from 0.20 to 0.50 for general conformation,
- from 0.01 to 0.15 for reproduction traits (kidding interval, age at first kidding, artificial insemination fertility),
- from -0.15 and -0.40 for somatic cell counts.
EBVs and reliabilities
For dairy animals, because of the low accuracy of breeding values, only males (and especially artificial insemination males) evaluated from the longevity data of their daughters, have EBVs that can be used for selection. A minimum number of daughters culled per sire is required to reach a sufficient accuracy. The consequence is that the AI males get their first longevity EBV quite late in their life. Survival analysis models, because they consider censored data (living daughters), enable better accuracy and thus, an earlier EBV for AI males.
Other strategies are possible to increase the accuracy of functional longevity EBVs:
- introduce genomic information in the genetic evaluation
- use a multiple trait model, including both functional longevity and other traits considered as predictors of longevity listed below.
Given the low heritability of survival traits, the fact that it is expressed late in life (at death or culling), the trait becomes accurate enough when sufficient information on culling or reproduction/lactation is available. It is necessary to enhance direct evaluations by indirect information coming from early predictors. Some relevant predictors are listed below:
- Morphological traits, such as general conformation or udder morphology (especially in dairy species),
- Reproduction traits (fertility, lambing/kidding interval, age at first lambing/kidding, pregnancy scan results, …),
- Udder health, and particularly milk somatic cell count,
- Resistance to disease such as resistance to parasites or to footrot,
- Traits related to feet and legs, such as lameness or twisted or bowed legs, closed or opened hocks,
- Serum immunoglobulin concentration in the early life (Ithurbide et al, 2022a),
- Maturity (dairy species) that can be defined as the ability to maintain a good level of production over the parities, independently of the level of production on the whole lifetime (equivalent of a persistency, but over the lactations and not over the test-days) (Arnal et al, 2022),
- Milk metabolites (Ithurbide et al, 2022b)
- Body tissue mobilisation (McLaren et al., 2023). It was demonstrated that ewe tissue mobilisation was genetically associated with ewe fertility and productive longevity (such as pregnancy scan result, foetal loss from scan to lambing, lamb loss from lambing to weaning, number of lambs weaned). It is made possible by collecting body condition score (BCS) data throughout the reproductive cycle (e.g. pre-mating, pregnancy scan, pre lambing, mid lactation, weaning) and calculating gain or loss of BCS between physiological stage.
These predictors are linked to longevity traits. An unfavourable udder shape, reproduction disorders, a susceptibility to a given disease or a low maturity may lead to involuntary culling and therefore a low longevity of the animal. Few genetic correlations have been published but correlations between EBVs show favourable correlations between these predictors and longevity.
Longevity traits, once evaluated, either in linear or survival analysis model, may be combined with the longevity traits in a multi-trait evaluation, to incorporate the information from early predictors.
A full multiple trait evaluation is not feasible in large datasets. Therefore, approximate strategies must be used, such as considering records adjusted for all non-genetic effects in linear models (yield deviation or daughter yield deviation, other type of pseudo records), or sub-indices incorporating traits that are linked together e.g. pulling together data on footrot, mastitis and parasite resistance could be considered together in a ‘health’ sub-index.
Culling causes
Even though the knowledge of the causes of culling is not necessary to generate a phenotype of longevity and an EBV of functional longevity, the knowledge of the causes of culling, through an observation based on a sufficient panel of flocks/herds, and repeated each year, may give relevant information on the hierarchy and the evolution of the culling causes. It may also enable better understanding of the strategies of culling by farmers leading to better modelling of functional longevity.
Culling causes may be collected with different levels of precision, from a general group of causes to a precise cause, through intermediate information.
In sheep as in goat, the following group of culling causes may be collected:
- Udder health (mastitis)
- Udder morphology
- Production ability
- Respiratory disorders
- Reproduction disorders
- Digestive disorders
- Nervous disorders
- Musculoskeletal disorders
- Skin disorders
- Conformation
- General condition
- Age
- Behaviour
- Accident
- Other ailments (e.g. sudden death, brucellosis, intoxication, fever …)
- Voluntary culling
Each group may be completed with sub-group or precise cause. Below are two examples, first for udder health (table 2), second for reproduction disorders (table 3).
Table 2. Detailed categorisation of udder health culling causes
| Group | Sub-group | Specific cause |
| Udder health (mastitis) | Gangrenous mastitis | Gangrenous mastitis |
| Brief mastitis | ||
| Characteristic symptoms | Mastitis | |
| Clinical mastitis | ||
| Mastitis during suckling | ||
| Coliform mastitis | ||
| Listeria mastitis | ||
| Mastitis before lambing/kidding | ||
| Agalactia mastitis | ||
| Functional symptoms | Blood in the milk | |
| Chronic mastitis, palpation | induration of the udder | |
| Bumps in the udder | ||
| Nodules | ||
| Mammary abcess | ||
| Saggy udder | ||
| Visna mastitis | ||
| Unbalanced udder | Milk in one side | |
| Unbalanced udder | ||
| Subclinical | Subclinical mastitis | |
| Somatic cell count (SCC) and California mastitis test– CMT | ||
| Other | Other |
Table 3. Detailed categorisation of reproduction disorders culling causes
| Group | Sub-group | Specific cause |
| Reproduction disorders | Fecundity | Open + infertile |
| Lately fertile, out of season | ||
| Ram infertile | ||
| Gestation | Abortion | |
| Vagina or rectal prolapse | ||
| Pregnancy toxaemia | ||
| Difficult gestation | ||
| Early abortion | ||
| Late abortion | ||
| Lambing/kidding | Difficult lambing/kidding | |
| Caesarean | ||
| Uterus inversion | ||
| Infection during lambing/kidding | ||
| Vagina or rectal prolapse | ||
| non deliverance | ||
| Acute metritis | ||
| Chronic metritis | ||
| Miscellaneous | Reproduction disorders | |
| Vaginal sponge infection | ||
| Hermaphrodite | ||
| Various | ||
| Male: testicles | 1 testicle | |
| Small testicles | ||
| Abscess | ||
| Contagious epididymitis | ||
| Male: penis | Urinary gravel | |
| Wound | ||
| Phimosis |
Acknowledgements
We gratefully acknowledge the contributions to these lifetime resilience guidelines by the following people:
- Joanne Conington, SRUC, the UK
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
- Isabelle Palhière, INRAE, France
- Jean-Michel Astruc, IDELE, France
- Carolina Pineda Quiroga, NEIKER, France
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Arnal M., Palhiere I., Clément V. (2022). Maturity, a new indicator to improve longevity of Saanen dairy goats in France. Proceedings of 12th World Congress on Genetics Applied to Livestock Production (WCGALP), Jul 2022, Rotterdam, Netherlands. doi:10.3920/978-90-8686-940-4_738.
Brotherstone, S., Veerkamp, R. F. and Hill, W. G. (1997). Genetic parameters for a simple predictor of the lifespan of Holstein-Friesian dairy cattle and its relationship to production. Animal Science 65: 31-37.
Buisson D., J.M. Astruc, L. Doutre, I. Palhière. Toward a genetic evaluation for functional longevity in French dairy sheep breeds. Proc 12th WCGALP, 2022
Castañeda-Bustos, V. J., Montaldo, H. H., Torres-Hernández, G., Pérez-Elizalde, S., Valencia-Posadas, M., Hernández-Mendo, O., & Shepard, L. (2014). Estimation of genetic parameters for productive life, reproduction, and milk-production traits in US dairy goats. Journal of Dairy Science, 97(4), 2462-2473.
Castañeda-Bustos, V. J., Montaldo, H. H., Valencia-Posadas, M., Shepard, L., Pérez-Elizalde, S., Hernández-Mendo, O., & Torres-Hernández, G. (2017). Linear and nonlinear genetic relationships between type traits and productive life in US dairy goats. Journal of Dairy Science, 100(2), 1232-1245.
Conington, J., Bishop, S. C., Grundy, B., Waterhouse, A., & Simm, G. (2001). Multi-trait selection indexes for sustainable UK hill sheep production. Animal Science, 73(3), 413-423.
Conington, J., Bishop, S.C., Waterhouse, A. and Simm, G. (2004). A bio-economic approach to derive economic values for pasture-based sheep genetic improvement programmes. Journal of Animal Science 82: 1290-1304. https://doi.org/10.2527/2004.8251290x
Ducrocq, V. (2001). A Two-Step Procedure to get Animal Model Solutions in Weibull Survival Models Used for Genetic Evaluations on Length of Productive Life. Interbull Bulletin, vol.27, pp.147-152
Ducrocq, V. (2005). An Improved model for the French genetic evaluation of dairy bulls on length of productive life of their daughters. Animal Science, 80(3), 249-256.
Geddes, L., Desire, S., Mucha, S., Coffey, M., Mrode, R. and Conington, J. (2018). Genetic parameters for longevity traits in UK dairy goats. IN: Proceedings of the World Congress on Genetics Applied to Livestock Production, Volume Species - Caprine: 547.
Ithurbide, M., Huau, C., Palhière, I., Fassier, T., Friggens, N. C., & Rupp, R. (2022a). Selection on functional longevity in a commercial population of dairy goats translates into significant differences in longevity in a common farm environment. Journal of Dairy Science, 105(5), 4289-4300.
Ithurbide, M., Wang, H., Huau, C., Palhière, I., Fassier, T., Pires, J. & Rupp, R. (2022b). Milk metabolite profiles in goats selected for longevity support link between resource allocation and resilience. In Proceedings of 12th World Congress on Genetics Applied to Livestock Production (WCGALP) pp. 276-279
McLaren A, Lambe, N R and Conington J. (2023). Genetic associations of ewe body condition score and lamb rearing performance in extensively managed sheep. 105336. Livestock Science September 2023 https://doi.org/10.1016/j.livsci.2023.105336
Palhière I., C. Oget, R. Rupp, Functional longevity is heritable and controlled by a major gene in French dairy goats, 11th WCGALP, Auckland, Nouvelle-Zelande, 11-16 février 2018
Pineda-Quiroga, C., Ugarte, E. (2022). An approach to functional longevity in Latxa dairy sheep. Livestock Science 263, 105003
Sasaki, O, (2013), Estimation of genetic parameters far longevity traits in dairy cattle: A review with focus o n the characteristics of analytical models, Animai Science Journal, 84(6), 449-460,
SMARTER Deliverable 2,2 - "New breeding goals far lifetime resilience far materna!sheep breeding programmes"
Guidelines on survival recording of foetus and young in sheep and goats
Change Summary
| Date of change | Nature of Change |
| October 2024 | First version |
| December 2024 | Tracked change revisions by MS |
Introduction and scope
Introduction
Foetal and young survival are parameters linked to neonatal vigour scores, maternal and young behaviours, stress responses, immunity transfer and traits related to dam fertility and longevity. Minimising mortality, either in utero (e.g., embryo/foetus) or pre-weaning, are crucial to profitable small ruminant production systems. Survival depends on an interaction between the environment and behaviour of both, the ewe and the lamb. Ewes must give birth without complications and provide reliable source of colostrum along with mothering environment. Lamb must adapt to the extra-uterine environment, thermoregulate and be able to stand and suckle in a reasonably short period after birth (Brien et al., 2014; Plush et al., 2016). Despite this, pre- weaning survival in many species is far from ideal (Binns et al., 2002; Yapi et al., 1990, Chaarani et al., 1991, Green and Morgan, 1993, Nash et al., 1996). This can be particularly worse in small ruminant production systems which are typically more extensive and therefore prevailing weather conditions can be an additional stressor as well as predators. Moreover, the poly-ovulatory nature of species such as sheep and goats also predisposes such species to greater foetal and pre-weaned young losses (Scales et al., 1986).
Litter size can be determined using trans-abdominal ultrasonography of the uterine horns at ideally 40-70 days post-fertilisation. Good accuracy in determining foetal number has been reported from trans-abdominal ultrasonography (Taverne et al., 1985). The number of young eventually born can then be used to assess foetal loss since the time of scanning. At birth, young survival is usually based on dead or not in the first 24 h post-birth while stillborn individuals or those dead within 24 hours are usually defined as failed to survive. Young survival can also be considered as different age group categories until weaning – for example from 1 day to 7 days of age. Young animals (i.e., < 7 days) are greatest at risk of mortality (Binns et al., 2002) and tend to die of exposure to hypothermia, starvation, septicaemia, or repercussions from trauma suffered at birth.
Scope
The aim of the present section is to define approaches for the definition of foetal and lamb survival as well as the data editing and downstream analyses (including statistical models).
Definition, terminology, rationale
A plethora of different definitions exist depending on whether defined at the level of the individual (i.e., binary trait) or that of the litter. A non-exhaustive list is given below.
Foetal survival (at an individual level):
- Can be defined as a binary trait of 0 (died between scanning and birth) or 1 (survived between scanning and birth). A dummy ID for the dead foetus would need to be constructed but the parentage would still potentially be known (especially if generated from AI).
Foetal survival (at a litter level):
- Whether or not some foetal mortality has occurred defined as a binary trait (i.e., the number of individuals born is less than the number scanned in utero)
- Number of individual foetuses scanned alive (along with gestational age)
- Number of foetuses scanned minus the number that were born (dead or alive) – this is a measure of foetal mortality as opposed to survival and assumes stillborn young are considered in the definition of a young survival trait. It is a count trait
- The number of young born divided by the number of foetuses scanned (this is mortality rate figure but per little with a penalty on losses for smaller litter sizes).
Young survival (at an individual level):
- Can be defined as a binary trait of 0 (dead within 24 hours of birth) or 1 (alive after 24 hours of birth). The dead animal would need to receive an ID and can, of course, be genotyped to verify parentage (but also used for downstream genomic analyses discussed later).
Young survival (at a litter level):
- Number of lambs born alive (NLBA)
- Number of lambs dead within 24 hours of birth
- Number of lambs dead within 24 hours of birth divided by the total number of lambs born
Recording survival of foetuses and young in small ruminant
In all instances, accurate data is crucial. Data should be collected on the animal/dam itself (dead or alive) but also potential confounding effects that could be considered for inclusion in the statistical model as fixed effects. Examples include contemporary group (e.g., flock-date of scanning, flock-year-season of birth (for each NLB separately), ewe parity, litter size). Ideally also all individuals should be genotyped. Because the heritability of foetal or young animal mortality in small ruminants is relatively low (<0.1; Safari et al., 2005; Brien et al., 2014), a large number of records are required to achieve accurate genetic/genomic evaluations. Care should also be taken when interpreting the scoring (and the following genetic evaluations), some jurisdictions may record mortality rather than survival or may record mortality but propose genetic evaluations as survival (i.e., positive value is favourable).
Pregnancy scanning records
Ideally scanning should be undertaken 40 to 70 days post-fertilisation. This may be possible to (easily) achieve where extensive AI has been used but, otherwise, should ideally be 30 days after the last female has been marked as been served by natural mating. Skilled operators should be able to determine the number of foetuses from 30 to 100 days of gestation; usually only one operator will scan a flock on a given day so will be confounded with flock-date of scanning contemporary group. If AI is solely used or if single sire mated, then the parentage of the foetus should be known; if mob mated or single sire mated at AI, then superfecundation could cause a discrepancy in recorded sire.
Young survival
Young survival can be defined at birth, ideally as a binary trait as to whether the animal was born stillborn or died within 24 hours (survival = 0) or was still alive 24 hours after birth (survival = 1). If information is also available on the reason for death (i.e., autopsy results) then, where sufficient data exists for any one ailment, it could be analysed separately as separate traits. This could be particularly important for generating separate genetic evaluations for the main diseases thereby not only possibly increasing the heritability through more accurate data, but also provide genetic evaluations specific to individual ailments which could enable more selection pressure on these traits in situations where they are more impactful. Ideally a genotype of the dead animal should be generated. Any obvious external defects should be noted.
Ancillary information
Having ancillary information coinciding with an event is useful for several reasons:
- For helping data editing (e.g., comparing actual birth date to expected birth date based on recorded service information)
- For adjustment in the statistical model (e.g., dam parity)
- Understanding the risk factors associated with survival
- Enabling more precise estimates of correlations with other performance traits by having information on multiple features from the same animal
- Adjusting for possible selection in multi-trait genetic evaluation models
Possible ancillary information can be divided into those associated with 1) the past of prevailing environmental conditions, 2) the dam (or sire), or 3) the individual. Examples include:
1. Environment:
- Weather related factors (rainfall, temperature, wind including direction)
- Flock
- Date of scanning or date of birth
2. Dam
- Parity
- Age
- Breed
- Genotype
- Litter size
- Mating type (i.e., AI versus natural)
- Body condition score (change) and live-weight (change)
- Mothering ability
- Colostrum quality and yield
3. Individual
- Days since service (for foetal survival trait)
- Birthing difficulty
- Birth weight
- Gender
- Genotype
- Sire
- Autopsy results if possible
Use for genetic analysis / genetic evaluation
Data editing and statistical modelling
In order to estimate contemporary group effects well, the larger the contemporary group, the better the group estimates. Therefore, imposing a minimum contemporary group size prior to data analysis should be considered as should good genetic connectedness with other contemporary groups. Genetic connectedness can be an issue with small ruminant populations in particular, especially where natural mating prevails.
Data editing
Foetal survival - Each flock-scanning date can be firstly investigated at a macro level to measure ultrasound quality control. Simple cross-references between the number of females with scanning data versus those presented as well as the ID numbers of both is useful to ensure all data were properly recorded. High foetal mortality rates could simply be indicative of high foetal loss (e.g., abortions due to causes like chlamydial and toxoplasma) as well as poor operator competence – assessing the rate for individual operators across flocks (and time) could be useful to assess operator proficiency. A high proportion of litters where the number of young born (dead or alive) exceeds that recorded at scanning suggests a poor accuracy of recording. It should be considered to discard the data from that date but also to investigate the operator in more detail across other flocks, and irrespective, the scanning results from that litter at least should be discarded. The proportion of scanned litters with >3 detected foetuses should also be calculated; depending on the expected prolificacy of the animals (e.g., breed), then the appropriate editing of either the individual data points or the date in its entirety should be assessed.
Young mortality - A high incidence of young mortality per contemporary group could simply be a consequence of some underlying issue (e.g., predation, disease) or indeed a high fecundity rate; a low incidence of young could be indicative of a good stock person. Therefore, it can be difficult to distinguish between high and low quality data. Using guaranteed high quality and reliable data, it is possible to estimate the expected distribution of the incidence of young animal mortality for different population strata such as flock size, ewe age, breed, litter size. Using these distributions, the probability that the mean mortality for a contemporary group fits this distribution can be estimated and a decision made as to whether or not to include the data in the downstream analyses.
Statistical modelling
Lamb survival is a complex trait influenced by direct genetic, maternal genetic, and environmental effects. Due to discrete expression of phenotype (dead or alive: 0 or 1) it is described as a threshold trait (Falconer, 1989) that violates the assumption of normality, and therefore linear models are theoretically not appropriate for the analysis. However, examples from the literature analysed survival data and reported that linear models were marginally more accurate at predicting missing phenotypes than were logit-transformed alternatives and are convenient for interpretation on the observed scale (Matos et al., 2000; Everett-Hincks et al., 2014; Cloete et al. 2009; Vanderick et al., 2015;).
Random effects considered in the statistical model are direct and maternal genetic effects and maternal permanent environment across parities. A litter permanent environmental effect should also be considered as a random effect where the trait is that of the individual (and not the ewe). Traditionally, relationships were accounted for though the pedigree data, however this can often now be supplemented with genome-wide genotype information to generate a H matrix (i.e., combines genomic and ancestry information). Whether the estimation of these additional covariance components improve the fit to the data can be deduced by a likelihood ratio test but ideally a metric such as the AIC or BIC to account for the increased complexity of the model.
The choice of environmental factors included in the model will depend on the population being studied and considers the following fixed effects:
- Contemporary group (e.g., flock-date of scanning for foetal survival and flock-year-season of birth or flock-year-season-birth rank of birth)
- Lamb gender (may not be possible for foetal survival trait)
- Dam parity
- Mating type (i.e., AI versus natural)
- Dam age nested within parity
- Day of gestation (for foetal survival) if available or defined as a categorical variable
- Litter size (at scanning or birth) or birth type (single and multiple)
- Heterosis and recombination loss of the dam and foetus/young
- Inbreeding coefficient of the dam and foetus/young
- Age of the sire
- Breed composition of the dam and foetus/young
Adjusting for the effects such as dystocia or birth weight, may not be appropriate in the statistical model for young survival as they are likely to be genetically correlated with survival and thus may remove some of the true genetic variance – nonetheless, the eventual decision will be based on the genetic evaluation system employed and how the economic value on the traits within the overall breeding objectives are constructed.
Genomic association analyses
Where genotypes are available, then a genome-wide association study (or candidate gene study) can be undertaken (Esmaeili-Fard et al., 2021). Although it is not possible to have the genotype of the aborted foetus, it could still be possible to undertake a genomic analysis especially by focusing on the genotype/haplotype of the living animals versus the expectation based on the genotype/haplotype of the parents (Ben Braiek et al., 2021).
Acknowledgements
We gratefully acknowledge the contributions to these recording of survival of foetus and young guidelines by the following people:
- Donagh Berry, TEAGASC, Ireland
- Joanne Conington, SRUC, the UK
- Maxime Ben Braiek, INRAE, France
- Arnaud Delpeuch, IDELE, France
- Marija Špehar, Centre for Livestock Breeding Zagreb, Croatia
This work received funding from the European Unions’ Horizon 2020 Research & Innovation program under grant agreement N°772787—SMARTER.
References
Ben Braiek, M., Fabre, S., Hozé, C., et al. (2021). Identification of homozygous haplotypes carrying putative recessive lethal mutations that compromise fertility traits in French Lacaune dairy sheep. Genet. Sel. Evol. 53:41. https://doi.org/10.1186/s12711-021-00634-1
Binns, S.H., I.J.Cox, S. Rizvi, L.E.Green. (2002). Risk factors for lamb mortality on UK sheep farms. Prev.Vet. Med.. 52:287-303.
Brien, F.D., Cloete, S.W.P., Fogarty, N.M., Greeff, J.C., Hebart, M.L., Hiendleder, S., Hocking Edwards, J.E., Kelly, J.M., Kind, K.L., Kleeman, D.O., Plush, K.L., Miller, D.R (2014). A review of genetic and epigenetic factors affecting lamb survival. Anim. Prod. Sci. 54:667–693.
Chaarani, B., Robinson, R.A., Johnson, D.W. (1991). Lamb mortality in Meknes Province (Morocco). Prev. Vet. Med. 10:283-298.
Cloete, S.W.P., Misztal, I., Olivier, J.J. (2009). Genetic parameters and trends for lamb survival and birth weight in a Merino flock divergently selected for multiple rearing ability. J. Anim. Sci. 87:2196–2208. doi:10.2527/jas.2008-1065.
Esmaeili-Fard, S.M., Gholizadeh, M., Hafezian, S.H., Abdollahi-Arpanahi, R. (2021) Genes and Pathways Affecting Sheep Productivity Traits: Genetic Parameters, Genome-Wide Association Mapping, and Pathway Enrichment Analysis. Front. Genet. 12:710613. doi:10.3389/fgene.2021.710613.
Everett-Hincks, J.M., Mathias-Davis, H.C,, Greer, G.J., Auvray, B.A., Dodds, K.G. (2014). Genetic parameters for lamb birth weight, survival and deathrisk traits. J. Anim. Sci. 92:2885–2895. doi:10.2527/jas.2013-7176.
Falconer, D.S. (1989). Introduction to Quantitative Genetics.’ (Longmans Green/John Wiley & Sons: Harlow, Essex, UK).
Green, L.E., Morgan, K.L. (1993). Mortality in early born, housed lambs in south-west England. Prev. Vet. Med. 17:251-261.
Matos, C.A.P., Thomas, D.L., Young, L.D., Gianola, D. (2000). Genetic analyses of lamb survival in Rambouillet and Finnsheep flocks by linear and threshold models. Anim. Sci. 71:227–234. doi:10.1017/S1357729800055053.
Nash, M.L., Hungerford, L.L., Nash, T.G., Zinn, G.M. (1996). Risk factors for perinatal and postnatal mortality in lambs. Vet. Rec. 139:64-67.
Plush, K.J., Brien, F.D., Hebart, M.L., Hynd, P.I. (2016). Thermogenesis and physiological maturity in neonatal lambs: a unifying concept in lamb survival. Anim. Prod. Sci. 56:736–745. https://doi.org/10.1071/AN15099.
Safari, E, Atkins, K.D., Fogarty, N.M., Gilmour, A.R (2005). Analysis of lamb survival in Australian Merino. Proceedings of the Association for the Advancement of Animal Breeding and Genetics. 16:28–31.
Scales, G. H., Burton R. N., Moss, R. A. (1986). Lamb mortality, birthweight, and nutrition in late pregnancy. N. Z. J. Agric. Res. 29:1.
Taverne, M.A.M. Lavoir, M.C., van Oord R., van der Weyden, G.C. (1985) Accuracy of pregnancy diagnosis and prediction of foetal numbers in sheep with linear‐array real‐time ultrasound scanning. Vet. Q. 7:(4)256-263, DOI: 10.1080/01652176.1985.9693997.
Vanderick, S., Auvray, B., Newman, S.A., Dodds, K.G., Gengler, N., EverettHincks, J.M. (2015). Derivation of a new lamb survival trait for the New Zealand sheep industry. J. Anim. Sci. 93:3765–3772. doi:10.2527/jas.2015-9058.
Yapi, C.V., Boylan, W.J., Robinson, R.A. (1990). Factors associated with causes of preweaning lamb mortality. Prev. Vet. Med., 10:145-152.
The technical references (papers cited or used) are documented in each piece of recommendations.
