Section 10 – Identification Device Certification: Difference between revisions
No edit summary |
|||
| Line 216: | Line 216: | ||
= Procedure 1: Conformance of Transponders = | = Procedure 1: Conformance of Transponders = | ||
== Foreword == | |||
ISO 24631 defines the test protocols for evaluating and verifying both the conformance (ISO 24631-1) and performance (ISO 24631-3) of RFID devices, and ISO 11784 defines the code structure. Only those results emanating from accredited and RA-approved test centres are recognized. | |||
== Introduction == | |||
ISO 11784 and ISO 11785 cover four RFID device types used for animal identification: | |||
# Injectables: a small transponder able to be injected into an animal's body. The transponder is encapsulated in a biocompatible and non porous material, e.g. glass. | |||
# Ear tag: a plastic covered transponder able to be fixed to an animal's ear using a locking mechanism which prevents the device from being removed without damaging it and rendering it unusable. | |||
# Ruminal bolus: a transponder placed into a high specific gravity container orally administered to a ruminant animal where the device remains in the rumen of the animal due its high specific gravity which prevents its passing through the animal's digestive system. | |||
# Tag attachment: a transponder covered by a primary protection layer but without its own locking system and is used only as an attachment to a visual ear tag or to another means of external animal identification, e.g. leg tag, collar, etc. | |||
The tests managed by ICAR as RA are recognised by the Federation of European Companion Animals Veterinary Association (FECAVA) and WSAVA (World Small Animal Veterinarian Association) and as such can be applied to companion animals also. | |||
The fee for all tests will be borne by the applicant. | |||
== References == | |||
ISO 11784 Agricultural equipment - Radio frequency identification of animals - Code structure | |||
ISO 11785 Agricultural equipment - Radio frequency identification of animals - Technical concept | |||
ISO 3166 Codes for the representation of names of countries and their subdivisions | |||
The latest version of ISO Standards will always apply and these Standards can be downloaded from the ISO website (https://www.iso.org/store.html). | |||
== Procedures for verifying the ISO conformance of transponders == | |||
=== Application === | |||
A manufacturer can apply for: | |||
# A full test; or | |||
# A limited test; or | |||
# A listing update. | |||
'''A. A full test is mandatory in the following cases:''' | |||
# When a non-RA registered manufacturer applies for a test. | |||
# When a RA registered manufacturer uses a new silicon chip (Integrated Circuit) or implements new technology (HDX or FDX-B) in the transponder; | |||
# When a RA registered manufacturer changes the coil technology (ferrite coils vs. air coils). | |||
'''B. A limited test is applicable in the following cases:''' | |||
# When a RA registered manufacturer inserts an ICAR certified transponder into a different primary transponder package. | |||
# When a RA registered manufacturer uses the silicon chip of an ICAR certified transponder with different coil dimensions. | |||
# When a RA registered manufacturer inserts an ICAR certified transponder with its original primary packaging into a different secondary packaging, e.g. a glass transponder into a bolus or a glass transponder into an ear tag. | |||
'''C. A listing update is applicable in the following case:''' | |||
# When a RA registered manufacturer intends to use an ICAR certified transponder without any modification. In this case the applicant must deliver a copy of the original test report along with a written confirmation from the ICAR registered manufacturer who originally submitted the transponder under question for certification by ICAR. | |||
To apply for an ISO transponder conformance test, the manufacturer has to complete the test application form given in Appendix A1 (Application for RFID transponder Conformance test (ISO 24631-1)) which is available [http://www.icar.org/wp-content/uploads/2016/03/Annex-A1-form-Section-10-Guidelines.pdf here]. | |||
The completed application must be emailed in PDF format to the ICAR secretariat at [mailto:manufacturers@icar.org manufacturers@icar.org]. | |||
The manufacturer may choose their preferred ICAR accredited test centre. The manufacturer is required to submit to the test centre: | |||
# 50 transponders for a full test, or | |||
# 10 transponders for a limited test, or | |||
# 10 transponders for a listing update. | |||
The submitted transponders must have the ICAR test code of 999 or the existing manufacturer's code for a full test. The manufacturer can freely choose the transponder codes, but duplicate codes are not allowed. The manufacturer must provide a list of the transponder codes in decimal format. | |||
Every specimen in a batch submitted for RFID testing (ISO24631-1 and/or ISO24631-3) must contain identical internal electronic components (coil and other components). Mixing of technologies (integrated circuit, capacitors, coils) within a single batch is prohibited. Likewise, when an electronic identifier is approved by ICAR, based on the results of testing, all identifiers that are released for sale must contain the same components as the test specimens. If changes are made to an identifier model after approval, the test requirements of ISO24631-1 section 6.1 must be met. | |||
The test centre will test the transponders for compliance with ISO 11784 and ISO 11785. All tested transponders must be readable by the laboratory reference transceiver. The codes read by the laboratory reference transceiver must comply with ISO 11784 and the identification codes must be on the list of codes provided by the manufacturer. | |||
The test centre will prepare a confidential report of the test results and will send the report to the ICAR secretariat. For a limited test or a listing update, the test report will contain only a summary of the test results. | |||
The ICAR Secretariat will send the test report to the manufacturer and, in the case of a successful Conformance test result, an official ICAR letter of certification signed by the ICAR Chief Executive will also be sent to the manufacturer, with a copy to the ISO/TC23/SC19/WG3 Chair. | |||
ICAR as RA issues a product code for each type of transponder successfully tested, including the listing update. | |||
All electronic transponders submitted in an application will be kept by the test centre as reference transponders. | |||
ICAR as RA maintains a public register on the ICAR website which lists all products registered and ICAR certified. A photograph of the certified device is included in the listing. | |||
=== Conditions for the right to use an ICAR certificate for transponders (conformance test) === | |||
Upon successful completion of the Conformance test, ICAR will grant a device certificate valid for five years. | |||
The ICAR certification of a transponder confirms the transponder's compliance with the code structure and the technical concepts given in ISO 11784 and ISO 11785. | |||
The manufacturer must maintain a database register of all ICAR certified transponders sold. The manufacturer must require the initial purchasers of their ICAR certified transponders to also maintain a database register of their purchased product and require all subsequent purchasers to do the same until the transponder is applied to an animal. | |||
The ICAR certificate is valid only for the transponder successfully tested and certified by ICAR. A manufacturer must not utilise the ICAR certificate for a transponder: | |||
# Which is not manufactured by them; and / or | |||
# Which does not comply in all respects with the ICAR certificate, including (but not limited to): | |||
#* Maintaining identical packaging (both primary and secondary) of the certified transponder. | |||
#* Maintaining identical technology and manufacturer of the certified transponder. | |||
#* Maintaining the identical transponder to the certified transponder. | |||
# Which utilises the manufacturer code of another manufacturer; | |||
# Which is supplied to or intended to be supplied to a person ("the receiver") who will market the transponder as if manufactured by them, unless: | |||
#* The receiver has obtained ICAR registration under this process; and | |||
#* The transponder bears either the shared manufacturer code or the unshared manufacturer code of the receiver. | |||
Once the ICAR certification has been granted, the manufacturer will be responsible to: | |||
# Keep an accurate and detailed log of all changes to their product and this log must be available to ICAR upon request. This log must include details of in-house performance measurements and Quality Assurance testing showing the amended product has maintained or enhanced its quality and performance. | |||
# Submit the product for re-certification before the expiration of its current ICAR certification. The manufacturer must apply for re-certification not earlier than 6 months before the expiration of the certificate and no later than 5 months after the expiration of the certificate. | |||
# Understand that ICAR may take sample products from the marketplace and test its conformance against the conformance of the device the manufacturer originally submitted, should ICAR suspect a breach of the signed ICAR Code of Conduct or a product change that has not been subjected to the tests outlined in this document - Procedure 1, Section 10 ‘Conformance of Transponders with ISO standards’. | |||
Should the manufacturer fail to meet any or all of the above conditions for the use of the ICAR certificate, actions may be taken by ICAR in its role as Registration Authority for ISO according to the ISO standard 24631-1. | |||
In cases of disputes regarding the conditions listed above or the use of an ICAR certificate, the decision of ICAR as RA will be binding. | |||
ICAR as RA will distribute an advice notice regarding any manufacturer that distributes transponders in conflict with the certification procedure. | |||
Revision as of 10:38, 13 August 2024
Overview
Introduction
This Section provides a general introduction to the principles and procedures developed for testing and certification of animal identification devices by ICAR.
On June 22, 2007 ISO appointed ICAR as the Registration Authority (RA) competent to register manufacturer codes used in the radio frequency identification (RFID) of animals in accordance with ISO 11784 and ISO 11785.
ICAR has administrative procedures in place for testing the conformance of RFID devices with respect to ISO 11784 and ISO 11785. Only those results coming from accredited test centres are recognized. In addition, ICAR offers evaluations on various quality and performance features of those devices that are tested for conformance with 11784/11785, and these evaluations are also available for conventional plastic ear tags.
Scope
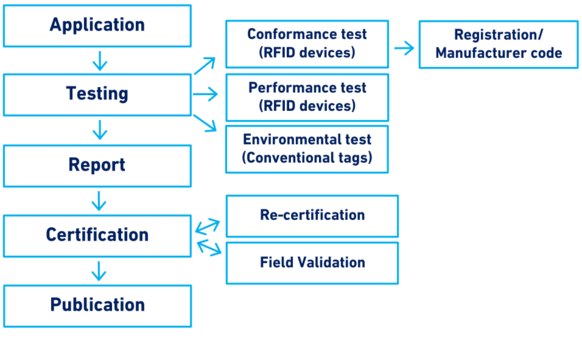
Figure 1 provides a summary of the main elements of this guideline.
In summary, section 10 of the ICAR Guidelines covers the testing and certification procedures, from the submission of the application by a manufacturer to the publication of the certification on the ICAR website, and the re-certification and/or sampling of the product.
Figure 1. Scope of Section 10: Testing and certification of animal identification devices
Application
The procedure for any type of test and certification starts with an application submitted by the manufacturer to the ICAR Secretariat. The Secretariat reviews the application, selects the test centre[1], issues an umbrella contract (only the first time that a test is requested) and issues the invoice that needs to be paid before the test starts. Financial transactions between manufacturers, test centres and ICAR are coordinated by the ICAR Secretariat. In order for the test to begin, the manufacturer sends all the necessary devices and accessories to the test centre. The devices and accessories remain the property of ICAR.
Testing
Testing of identification devices can be subdivided into four main categories as reported in Table 2.
RFID Conformance test (ISO 24631-1)
Conformance testing is required to demonstrate electronic transponders meet the specifications and standards in ISO 11784 and ISO 11785. The submission of identification devices to conformance testing is obligatory before they can be used in the official identification of animals.
Conformance tests are coordinated by the ICAR Secretariat. Acting as the RA on behalf of ISO, ICAR issues a Certificate of Conformance for RFID devices conforming with ISO 11784 and ISO 11785.
Details of the RFID Conformance test are described in Procedure 1, Section 10 ‘Conformance of RFID Transponders with ISO 11784 and ISO 11785’ available here.
RFID Performance test (ISO 24631-3)
Performance testing is an evaluation of the following characteristics of an RFID device: modulation amplitude, bit length stability, minimum activation field strength resonance frequency and amplitude voltage response (Vss). These RFID performance test results are not subject to pass or fail criteria but provide useful additional information on device behaviour when communicating with a reader. Acting as the RA on behalf of ISO, ICAR evaluates RFID devices through the RFID performance test and provides the report of the performance test to the manufacturer.
Device Composition and Environmental Performance test (ICAR)
ICAR offers a device composition and environmental performance test for both conventional and RFID external devices. The objective of these tests is to give extensive information on device durability and performance in diverse animal management conditions. Procedures will vary depending on the device type. ICAR shares the test report and ICAR certificate for devices in accordance with the specifications of the respective ICAR.
Details of the device composition and environmental performance test are described in Procedure 4, Section 10 ‘Testing of Conventional Plastic Ear Tags’ available here and Procedure 5, Section 10 ‘Testing of External RFID Devices’ available here.
Voluntary sampling of Animal Identification Devices
Voluntary sampling of Animal Identification Devices is a service for National Competent Authorities or other service users, other than manufacturers or their agents. The service is a quality verification service to ensure that devices available in the relevant market(s) remain compliant with the appropriate ISO and ICAR test protocols. Voluntary sampling does not lead to certification of the devices.
Details of the service are described in Procedure 6, Section 10 ‘Voluntary Sampling of Identification Devices’ available here.
Summary of Tests
Table 2 summarizes the categories of tests.
| Table 2. Categories for the testing of identification devices. | ||
| Test category | Test description | Link to test procedure |
| Conformance and Performance ISO 24631-1 | Conformance/performance test of transponder (incl. granting of manufacturer code) or transceiver | Procedure 1, Section 10
‘Conformance of Transponders with ISO standards’ Procedure 2, Section 10 ‘Granting of Manufacturer Code’ Procedure 3, Section 10 ‘Conformance of Transceivers with ISO standards’ |
| Composition and environmental performance – Conventional ear tags | Extended laboratory test | Procedure 4, Section 10
‘Testing of Conventional Plastic Ear Tags’ |
| Composition and environmental performance – External RFID devices | Extended laboratory test | Procedure 5, Section 10
‘Testing of External RFID Devices’ |
| Voluntary sampling | Partial test for certified devices available on the market. | Procedure 6, Section 10
‘Voluntary sampling of Identification Devices’ |
Test Centres
Testing is conducted by ICAR accredited test centres. Tests are contracted by the ICAR Secretariat to a specific test centre. The test centre is obliged to act according to the procedures laid down within the test protocols. In addition, all details associated with the testing phase, including the test results, must be kept strictly confidential. Test centres are regularly monitored by the ICAR Animal Identification Sub-Committee and their contact details are available here.
Manufacturer code
Following the first successful full conformance test, ICAR in its role as RA for ISO for the standards 11784/11785 – allocates to the manufacturer a code to be used only for products registered by ICAR. There are two types of manufacturer codes:
- Shared manufacturer code (900): can be granted to more than one manufacturer. A restricted range of identification codes is allocated to the registered product for exclusive use together with the shared manufacturer code.
- Unshared manufacturer code (901-998): can only be granted to one manufacturer following official proof that during two consecutive years the company has sold a minimum of one million (ICAR certified) transponders per year.
Note: the manufacturer code concerns only the certification of RFID devices. As regards conventional ear tags, ICAR allocates unique certification codes to the products that pass the Device composition and environmental performance test.
Report
Test centres prepare a confidential report of the test results and submit the report to the ICAR Secretariat. The Secretariat checks the report and forwards it to the manufacturer, together with the ICAR certificate in case of successful test. The report is also shared for information with the Animal Identification Sub-Committee.
Certification
The tests that lead to an ICAR certificate are:
- RFID Conformance test (ISO 24631-1).
- Device Composition and Environmental Performance test (ICAR).
Certificates are issued by the ICAR Secretariat and signed by the ICAR Chief Executive. They are sent to the manufacturer by e-mail. In the case of certificates of conformance, the Chair of the ISO/TC23/SC19/WG3 is copied so that ISO is informed about registered devices under the RA Agreement.
For other tests not subject to pass or fail criteria (e.g. Performance test), an official ICAR letter acknowledging the completion of the test is sent to the manufacturer.
Publication
All ICAR-certified devices are published on the ICAR website:
Devices whose certification has expired are removed from the webpage. A specific web page contains all the devices registered by ICAR in conformance with ISO standards 11784 and 11785. Devices listed in this webpage are never removed since the registration is valid for the lifetime of the device.
Table 3 summarizes the steps and responsibilities in the ICAR certification procedure.
| Table 3. Steps, actions and responsibilities in the ICAR certification procedure. | ||
| Step | Action | Responsibility |
| 1 | Application for testing of a device | Manufacturer or dealer of identification device |
| 2 | Acceptance of application, and issuance of umbrella contract and invoice | ICAR Secretariat |
| 3 | Testing and report compilation | ICAR test centres |
| 4 | Sharing of test results with the applicant | ICAR Secretariat |
| 5 | ICAR certification | ICAR Secretariat |
| 6 | Publication on the website | ICAR Secretariat |
Re-certification
After 5 years from the issuance of an ICAR certificate, the test can be repeated in order for the certification to be renewed for another 5 years. The device maintains its original product/certification code. The test protocols applied for the re-certification are:
- The limited test protocol for the RFID devices
- The preliminary assessment protocol for the conventional devices
Note: if the application for re-certification is submitted more than 5 years after the original certification, full test procedures are carried out.
The application process is the same as for any other tests. Once re-certified, the device remains on the ICAR website for another 5 years and the latest date of certification is indicated.
Voluntary sampling
At any given moment, Competent Authorities or other service users can apply for a sampling of certified devices found on the market. Devices are tested against the current ICAR standards and the results are compared with original or earlier results for the same devices. The test protocols used by the laboratories are:
- The limited test protocol for the RFID devices.
- The preliminary assessment protocol for the conventional devices.
The applicant may also request or specify additional test protocols, provided these are defined in other existing ISO or ICAR higher level test protocols.
It is required that the devices to be tested are collected from the local market stock by the applicant and not by the manufacturer.
Conditions for the use of ICAR certificates
- The conditions for the use of ICAR certificates are described in the respective procedures.
- If a device is certified by ICAR, the manufacturer may publish the certification of its device.
- ICAR certification does not guarantee that the device is suitable for all environments.
Note: A manufacturer must not use the ICAR logo for any purpose.
Appendices
Appendix A1. Application for RFID transponder Conformance test (ISO 24631-1) (link)
Appendix A2. Application for a manufacturer code allocation (link)
Appendix A3. Code of conduct (link)
Appendix A4. Application for RFID transponder Performance test (ISO 24631-3) (link)
Appendix A5. Application for RFID transceiver Conformance test (ISO 24631-2) (link)
Appendix A6. Application for RFID transceiver Performance test (ISO 24631-4) (link)
Appendix B1. Application for Device Composition and Environmental Performance test for conventional ear tags (link)
Appendix B2. Application for Device Change Notification (DCN) for conventional ear tags modified during the 5-year certification (link)
Appendix B3. Numbers for Reference Printing (link).
Appendix B4. Preliminary Test for Conventional Plastic Ear Tags (link).
Appendix B5. Laboratory Test for Conventional Plastic Ear Tags (link).
Appendix C1. Application for Device Composition and Environmental Performance test for external RFID devices (link)
Appendix C2. Application for Device Change Notification (DCN) for external RFID devices modified during the 5-year certification (link)
Appendix C3. Preliminary Test for External RFID Devices (link).
Appendix C4. Laboratory Test for External RFID Devices (link).
Appendix D1. Application for voluntary sampling of animal identification device (link) [1] Manufacturers also have the possibility to choose their preferred test centre.
Procedure 1: Conformance of Transponders
Foreword
ISO 24631 defines the test protocols for evaluating and verifying both the conformance (ISO 24631-1) and performance (ISO 24631-3) of RFID devices, and ISO 11784 defines the code structure. Only those results emanating from accredited and RA-approved test centres are recognized.
Introduction
ISO 11784 and ISO 11785 cover four RFID device types used for animal identification:
- Injectables: a small transponder able to be injected into an animal's body. The transponder is encapsulated in a biocompatible and non porous material, e.g. glass.
- Ear tag: a plastic covered transponder able to be fixed to an animal's ear using a locking mechanism which prevents the device from being removed without damaging it and rendering it unusable.
- Ruminal bolus: a transponder placed into a high specific gravity container orally administered to a ruminant animal where the device remains in the rumen of the animal due its high specific gravity which prevents its passing through the animal's digestive system.
- Tag attachment: a transponder covered by a primary protection layer but without its own locking system and is used only as an attachment to a visual ear tag or to another means of external animal identification, e.g. leg tag, collar, etc.
The tests managed by ICAR as RA are recognised by the Federation of European Companion Animals Veterinary Association (FECAVA) and WSAVA (World Small Animal Veterinarian Association) and as such can be applied to companion animals also.
The fee for all tests will be borne by the applicant.
References
ISO 11784 Agricultural equipment - Radio frequency identification of animals - Code structure
ISO 11785 Agricultural equipment - Radio frequency identification of animals - Technical concept
ISO 3166 Codes for the representation of names of countries and their subdivisions
The latest version of ISO Standards will always apply and these Standards can be downloaded from the ISO website (https://www.iso.org/store.html).
Procedures for verifying the ISO conformance of transponders
Application
A manufacturer can apply for:
- A full test; or
- A limited test; or
- A listing update.
A. A full test is mandatory in the following cases:
- When a non-RA registered manufacturer applies for a test.
- When a RA registered manufacturer uses a new silicon chip (Integrated Circuit) or implements new technology (HDX or FDX-B) in the transponder;
- When a RA registered manufacturer changes the coil technology (ferrite coils vs. air coils).
B. A limited test is applicable in the following cases:
- When a RA registered manufacturer inserts an ICAR certified transponder into a different primary transponder package.
- When a RA registered manufacturer uses the silicon chip of an ICAR certified transponder with different coil dimensions.
- When a RA registered manufacturer inserts an ICAR certified transponder with its original primary packaging into a different secondary packaging, e.g. a glass transponder into a bolus or a glass transponder into an ear tag.
C. A listing update is applicable in the following case:
- When a RA registered manufacturer intends to use an ICAR certified transponder without any modification. In this case the applicant must deliver a copy of the original test report along with a written confirmation from the ICAR registered manufacturer who originally submitted the transponder under question for certification by ICAR.
To apply for an ISO transponder conformance test, the manufacturer has to complete the test application form given in Appendix A1 (Application for RFID transponder Conformance test (ISO 24631-1)) which is available here.
The completed application must be emailed in PDF format to the ICAR secretariat at manufacturers@icar.org.
The manufacturer may choose their preferred ICAR accredited test centre. The manufacturer is required to submit to the test centre:
- 50 transponders for a full test, or
- 10 transponders for a limited test, or
- 10 transponders for a listing update.
The submitted transponders must have the ICAR test code of 999 or the existing manufacturer's code for a full test. The manufacturer can freely choose the transponder codes, but duplicate codes are not allowed. The manufacturer must provide a list of the transponder codes in decimal format.
Every specimen in a batch submitted for RFID testing (ISO24631-1 and/or ISO24631-3) must contain identical internal electronic components (coil and other components). Mixing of technologies (integrated circuit, capacitors, coils) within a single batch is prohibited. Likewise, when an electronic identifier is approved by ICAR, based on the results of testing, all identifiers that are released for sale must contain the same components as the test specimens. If changes are made to an identifier model after approval, the test requirements of ISO24631-1 section 6.1 must be met.
The test centre will test the transponders for compliance with ISO 11784 and ISO 11785. All tested transponders must be readable by the laboratory reference transceiver. The codes read by the laboratory reference transceiver must comply with ISO 11784 and the identification codes must be on the list of codes provided by the manufacturer.
The test centre will prepare a confidential report of the test results and will send the report to the ICAR secretariat. For a limited test or a listing update, the test report will contain only a summary of the test results.
The ICAR Secretariat will send the test report to the manufacturer and, in the case of a successful Conformance test result, an official ICAR letter of certification signed by the ICAR Chief Executive will also be sent to the manufacturer, with a copy to the ISO/TC23/SC19/WG3 Chair.
ICAR as RA issues a product code for each type of transponder successfully tested, including the listing update.
All electronic transponders submitted in an application will be kept by the test centre as reference transponders.
ICAR as RA maintains a public register on the ICAR website which lists all products registered and ICAR certified. A photograph of the certified device is included in the listing.
Conditions for the right to use an ICAR certificate for transponders (conformance test)
Upon successful completion of the Conformance test, ICAR will grant a device certificate valid for five years.
The ICAR certification of a transponder confirms the transponder's compliance with the code structure and the technical concepts given in ISO 11784 and ISO 11785.
The manufacturer must maintain a database register of all ICAR certified transponders sold. The manufacturer must require the initial purchasers of their ICAR certified transponders to also maintain a database register of their purchased product and require all subsequent purchasers to do the same until the transponder is applied to an animal.
The ICAR certificate is valid only for the transponder successfully tested and certified by ICAR. A manufacturer must not utilise the ICAR certificate for a transponder:
- Which is not manufactured by them; and / or
- Which does not comply in all respects with the ICAR certificate, including (but not limited to):
- Maintaining identical packaging (both primary and secondary) of the certified transponder.
- Maintaining identical technology and manufacturer of the certified transponder.
- Maintaining the identical transponder to the certified transponder.
- Which utilises the manufacturer code of another manufacturer;
- Which is supplied to or intended to be supplied to a person ("the receiver") who will market the transponder as if manufactured by them, unless:
- The receiver has obtained ICAR registration under this process; and
- The transponder bears either the shared manufacturer code or the unshared manufacturer code of the receiver.
Once the ICAR certification has been granted, the manufacturer will be responsible to:
- Keep an accurate and detailed log of all changes to their product and this log must be available to ICAR upon request. This log must include details of in-house performance measurements and Quality Assurance testing showing the amended product has maintained or enhanced its quality and performance.
- Submit the product for re-certification before the expiration of its current ICAR certification. The manufacturer must apply for re-certification not earlier than 6 months before the expiration of the certificate and no later than 5 months after the expiration of the certificate.
- Understand that ICAR may take sample products from the marketplace and test its conformance against the conformance of the device the manufacturer originally submitted, should ICAR suspect a breach of the signed ICAR Code of Conduct or a product change that has not been subjected to the tests outlined in this document - Procedure 1, Section 10 ‘Conformance of Transponders with ISO standards’.
Should the manufacturer fail to meet any or all of the above conditions for the use of the ICAR certificate, actions may be taken by ICAR in its role as Registration Authority for ISO according to the ISO standard 24631-1.
In cases of disputes regarding the conditions listed above or the use of an ICAR certificate, the decision of ICAR as RA will be binding.
ICAR as RA will distribute an advice notice regarding any manufacturer that distributes transponders in conflict with the certification procedure.
