Section 10 – Identification Device Certification
Overview
Introduction
This Section provides a general introduction to the principles and procedures developed for testing and certification of animal identification devices by ICAR.
On June 22, 2007 ISO appointed ICAR as the Registration Authority (RA) competent to register manufacturer codes used in the radio frequency identification (RFID) of animals in accordance with ISO 11784 and ISO 11785.
ICAR has administrative procedures in place for testing the conformance of RFID devices with respect to ISO 11784 and ISO 11785. Only those results coming from accredited test centres are recognized. In addition, ICAR offers evaluations on various quality and performance features of those devices that are tested for conformance with 11784/11785, and these evaluations are also available for conventional plastic ear tags.
Scope
Figure 1 provides a summary of the main elements of this guideline.
In summary, section 10 of the ICAR Guidelines covers the testing and certification procedures, from the submission of the application by a manufacturer to the publication of the certification on the ICAR website, and the re-certification and/or sampling of the product.
Figure 1. Scope of Section 10: Testing and certification of animal identification devices
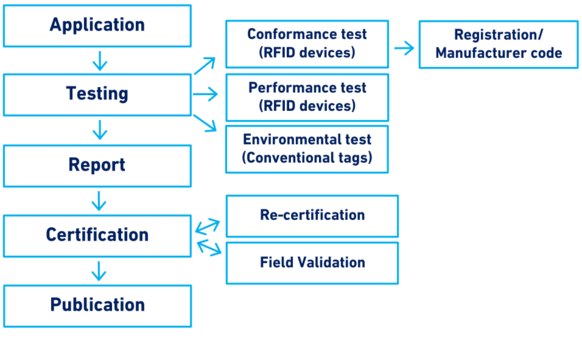
Application
The procedure for any type of test and certification starts with an application submitted by the manufacturer to the ICAR Secretariat. The Secretariat reviews the application, selects the test centre[1], issues an umbrella contract (only the first time that a test is requested) and issues the invoice that needs to be paid before the test starts. Financial transactions between manufacturers, test centres and ICAR are coordinated by the ICAR Secretariat. In order for the test to begin, the manufacturer sends all the necessary devices and accessories to the test centre. The devices and accessories remain the property of ICAR.
Testing
Testing of identification devices can be subdivided into four main categories as reported in Table 2.
RFID Conformance test (ISO 24631-1)
Conformance testing is required to demonstrate electronic transponders meet the specifications and standards in ISO 11784 and ISO 11785. The submission of identification devices to conformance testing is obligatory before they can be used in the official identification of animals.
Conformance tests are coordinated by the ICAR Secretariat. Acting as the RA on behalf of ISO, ICAR issues a Certificate of Conformance for RFID devices conforming with ISO 11784 and ISO 11785.
Details of the RFID Conformance test are described in Procedure 1, Section 10 ‘Conformance of RFID Transponders with ISO 11784 and ISO 11785’ available here.
RFID Performance test (ISO 24631-3)
Performance testing is an evaluation of the following characteristics of an RFID device: modulation amplitude, bit length stability, minimum activation field strength resonance frequency and amplitude voltage response (Vss). These RFID performance test results are not subject to pass or fail criteria but provide useful additional information on device behaviour when communicating with a reader. Acting as the RA on behalf of ISO, ICAR evaluates RFID devices through the RFID performance test and provides the report of the performance test to the manufacturer.
Device Composition and Environmental Performance test (ICAR)
ICAR offers a device composition and environmental performance test for both conventional and RFID external devices. The objective of these tests is to give extensive information on device durability and performance in diverse animal management conditions. Procedures will vary depending on the device type. ICAR shares the test report and ICAR certificate for devices in accordance with the specifications of the respective ICAR.
Details of the device composition and environmental performance test are described in Procedure 4, Section 10 ‘Testing of Conventional Plastic Ear Tags’ available here and Procedure 5, Section 10 ‘Testing of External RFID Devices’ available here.
Voluntary sampling of Animal Identification Devices
Voluntary sampling of Animal Identification Devices is a service for National Competent Authorities or other service users, other than manufacturers or their agents. The service is a quality verification service to ensure that devices available in the relevant market(s) remain compliant with the appropriate ISO and ICAR test protocols. Voluntary sampling does not lead to certification of the devices.
Details of the service are described in Procedure 6, Section 10 ‘Voluntary Sampling of Identification Devices’ available here.
Summary of Tests
Table 2 summarizes the categories of tests.
| Table 2. Categories for the testing of identification devices. | ||
| Test category | Test description | Link to test procedure |
| Conformance and Performance ISO 24631-1 | Conformance/performance test of transponder (incl. granting of manufacturer code) or transceiver | Procedure 1, Section 10
‘Conformance of Transponders with ISO standards’ Procedure 2, Section 10 ‘Granting of Manufacturer Code’ Procedure 3, Section 10 ‘Conformance of Transceivers with ISO standards’ |
| Composition and environmental performance – Conventional ear tags | Extended laboratory test | Procedure 4, Section 10
‘Testing of Conventional Plastic Ear Tags’ |
| Composition and environmental performance – External RFID devices | Extended laboratory test | Procedure 5, Section 10
‘Testing of External RFID Devices’ |
| Voluntary sampling | Partial test for certified devices available on the market. | Procedure 6, Section 10
‘Voluntary sampling of Identification Devices’ |
Test Centres
Testing is conducted by ICAR accredited test centres. Tests are contracted by the ICAR Secretariat to a specific test centre. The test centre is obliged to act according to the procedures laid down within the test protocols. In addition, all details associated with the testing phase, including the test results, must be kept strictly confidential. Test centres are regularly monitored by the ICAR Animal Identification Sub-Committee and their contact details are available here.
Manufacturer code
Following the first successful full conformance test, ICAR in its role as RA for ISO for the standards 11784/11785 – allocates to the manufacturer a code to be used only for products registered by ICAR. There are two types of manufacturer codes:
- Shared manufacturer code (900): can be granted to more than one manufacturer. A restricted range of identification codes is allocated to the registered product for exclusive use together with the shared manufacturer code.
- Unshared manufacturer code (901-998): can only be granted to one manufacturer following official proof that during two consecutive years the company has sold a minimum of one million (ICAR certified) transponders per year.
Note: the manufacturer code concerns only the certification of RFID devices. As regards conventional ear tags, ICAR allocates unique certification codes to the products that pass the Device composition and environmental performance test.
Report
Test centres prepare a confidential report of the test results and submit the report to the ICAR Secretariat. The Secretariat checks the report and forwards it to the manufacturer, together with the ICAR certificate in case of successful test. The report is also shared for information with the Animal Identification Sub-Committee.
Certification
The tests that lead to an ICAR certificate are:
- RFID Conformance test (ISO 24631-1).
- Device Composition and Environmental Performance test (ICAR).
Certificates are issued by the ICAR Secretariat and signed by the ICAR Chief Executive. They are sent to the manufacturer by e-mail. In the case of certificates of conformance, the Chair of the ISO/TC23/SC19/WG3 is copied so that ISO is informed about registered devices under the RA Agreement.
For other tests not subject to pass or fail criteria (e.g. Performance test), an official ICAR letter acknowledging the completion of the test is sent to the manufacturer.
Publication
All ICAR-certified devices are published on the ICAR website:
Devices whose certification has expired are removed from the webpage. A specific web page contains all the devices registered by ICAR in conformance with ISO standards 11784 and 11785. Devices listed in this webpage are never removed since the registration is valid for the lifetime of the device.
Table 3 summarizes the steps and responsibilities in the ICAR certification procedure.
| Table 3. Steps, actions and responsibilities in the ICAR certification procedure. | ||
| Step | Action | Responsibility |
| 1 | Application for testing of a device | Manufacturer or dealer of identification device |
| 2 | Acceptance of application, and issuance of umbrella contract and invoice | ICAR Secretariat |
| 3 | Testing and report compilation | ICAR test centres |
| 4 | Sharing of test results with the applicant | ICAR Secretariat |
| 5 | ICAR certification | ICAR Secretariat |
| 6 | Publication on the website | ICAR Secretariat |
Re-certification
After 5 years from the issuance of an ICAR certificate, the test can be repeated in order for the certification to be renewed for another 5 years. The device maintains its original product/certification code. The test protocols applied for the re-certification are:
- The limited test protocol for the RFID devices
- The preliminary assessment protocol for the conventional devices
Note: if the application for re-certification is submitted more than 5 years after the original certification, full test procedures are carried out.
The application process is the same as for any other tests. Once re-certified, the device remains on the ICAR website for another 5 years and the latest date of certification is indicated.
Voluntary sampling
At any given moment, Competent Authorities or other service users can apply for a sampling of certified devices found on the market. Devices are tested against the current ICAR standards and the results are compared with original or earlier results for the same devices. The test protocols used by the laboratories are:
- The limited test protocol for the RFID devices.
- The preliminary assessment protocol for the conventional devices.
The applicant may also request or specify additional test protocols, provided these are defined in other existing ISO or ICAR higher level test protocols.
It is required that the devices to be tested are collected from the local market stock by the applicant and not by the manufacturer.
Conditions for the use of ICAR certificates
- The conditions for the use of ICAR certificates are described in the respective procedures.
- If a device is certified by ICAR, the manufacturer may publish the certification of its device.
- ICAR certification does not guarantee that the device is suitable for all environments.
Note: A manufacturer must not use the ICAR logo for any purpose.
Appendices
Appendix A1. Application for RFID transponder Conformance test (ISO 24631-1) (link)
Appendix A2. Application for a manufacturer code allocation (link)
Appendix A3. Code of conduct (link)
Appendix A4. Application for RFID transponder Performance test (ISO 24631-3) (link)
Appendix A5. Application for RFID transceiver Conformance test (ISO 24631-2) (link)
Appendix A6. Application for RFID transceiver Performance test (ISO 24631-4) (link)
Appendix B1. Application for Device Composition and Environmental Performance test for conventional ear tags (link)
Appendix B2. Application for Device Change Notification (DCN) for conventional ear tags modified during the 5-year certification (link)
Appendix B3. Numbers for Reference Printing (link).
Appendix B4. Preliminary Test for Conventional Plastic Ear Tags (link).
Appendix B5. Laboratory Test for Conventional Plastic Ear Tags (link).
Appendix C1. Application for Device Composition and Environmental Performance test for external RFID devices (link)
Appendix C2. Application for Device Change Notification (DCN) for external RFID devices modified during the 5-year certification (link)
Appendix C3. Preliminary Test for External RFID Devices (link).
Appendix C4. Laboratory Test for External RFID Devices (link).
Appendix D1. Application for voluntary sampling of animal identification device (link) [1] Manufacturers also have the possibility to choose their preferred test centre.
